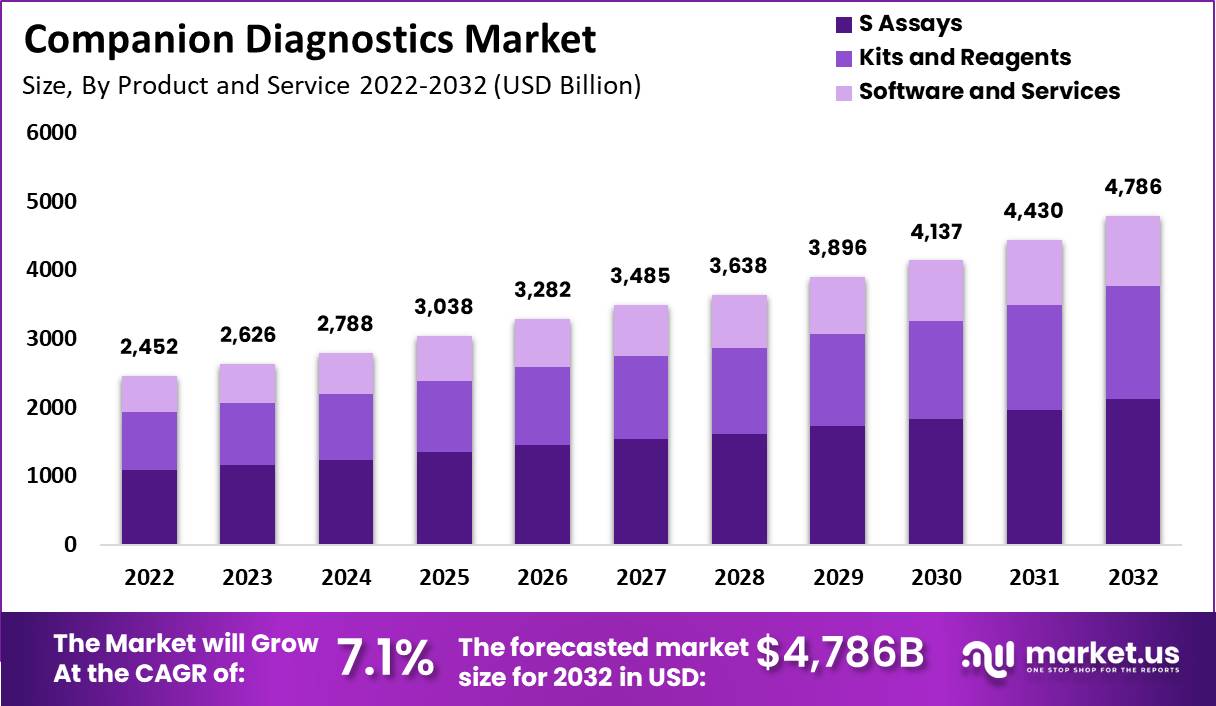
New York, April 10, 2023 (GLOBE NEWSWIRE) -- The global companion diagnostics market size is projected to surpass around USD 4,786 million by 2032 from USD 2,452 Million in 2022, and it is poised to register a CAGR of 7.1% from 2023 to 2032. Companion diagnostics is an advanced medical device used with therapeutic drugs to calculate the effect & convenience of medicines on a living human body. Companion diagnostics are developed concerning drugs for a selected group of patients based on their characteristics and how they respond to the therapy. This device helps the healthcare professional determine whether a treatment benefits the patient.
To get additional highlights on major revenue-generating segments, Request a global companion diagnostics market sample report at https://market.us/report/companion-diagnostics-market/request-sample/
Key Takeaway:
- By Product and Services, the assay segment has generated the highest revenue share during the forecast period (2023-2032).
- By Technology, the Polymerase Chain Reaction segment has dominated the Market and is growing at the fastest CAGR from 2023 to 2032.
- By Indication, increasing care for cancer and the participation of prominent players in innovative technology development dominated the segment.
- By End-User, Collaborations with Manufacturers Leading to the Significant Share of the Pharmaceutical & Biopharmaceutical Companies Segment.
- In 2022, North America dominated the Market with the highest revenue share of 43%.
- Europe held a 25% revenue share in 2022.
- Asia-Pacific will grow at the fastest CAGR during the forecast period (2023-2032).
Growing patient awareness for tailored therapy, increased number of chronic diseases, increased cases of allergies in patients, and a wide variety of applications of personalized medications due to adverse medication effects drive the development of personalized CDx, thereby propelling the Market's growth. Additionally, the advantages of CDx tests, such as high sensitivity, fast and accurate results in shorter time frames, and fundamental cost-effectiveness, contribute to the growth of the Companion Diagnostics Market.
Factors Affecting the Growth of the Global Companion Diagnostics Market
Several factors can affect the growth of the global companion diagnostics market. Some of these factors include:
- Increasing Demand for Cost-Effective Quality Care: Global companion diagnostics market is gaining popularity as they enable healthcare organizations to provide cost-effective quality care and access to the latest medical technologies and knowledge. This encourages healthcare organizations to innovate their core activities to healthcare devices, thus driving the Market's growth.
- Growing Need for Regulatory Compliance: Healthcare organizations increasingly focus on regulatory compliance and carry out activities such as Collaborations with Manufacturers, Leading to a Significant Share of the Pharmaceutical & Biopharmaceutical Companies Segment. This is expected to drive the growth of the global companion diagnostics market.
- Rise in Prevalence of Chronic Diseases: The rising prevalence of chronic diseases increases the demand for timely and accurate diagnosis and treatment. This is driving the need for the global companion diagnostics market.
- Growing Focus on Patient-Centric Care: Healthcare organizations increasingly focus on patient-centric care by providing personalized services and treatments. This is driving the demand for companion diagnostics devices, which is expected to drive the growth of the global companion diagnostics market.
To understand how our global companion diagnostics market report can bring a difference to your business strategy, Inquire about a brochure at https://market.us/report/companion-diagnostics-market/#inquiry
Top Trends in Global Companion Diagnostics Market
The next-generation sequencing technique is another key trend in the global companion diagnostics market. This test helps determine the number of genes responsible for cancer development, mainly done on the surgically removed tumor. Increasing demand for NGS technology is another major global companion diagnostics market trend. This technology gave high output in a short timeframe in healthcare organizations and focused on quality care to patients.
Market Growth
It is now accepted that different medications can produce shifting results due to genetics and genetic sequence. Understanding an individual's biomarkers or hereditary traits is essential for overseeing the 'right medication, at the right time, for the perfect person.' The biopharmaceutical and drug companies continue to work to develop patient-choice analysis systems for the first phases of medication enhancement to offer the appropriate treatments to the right customer. These support the growth of the global companion diagnostics market.
Regional Analysis
North America is expected to dominate the global companion diagnostics market during the forecast period. The region has a higher incidence of cancer and other chronic diseases in the area and increased adoption of advanced CDx tests. The European region is expected to grow positively during the forecast period. The rising demand for cost-effective healthcare services and the growing need for quality healthcare services are major factors driving the market growth in the region. The Asia Pacific region is projected to witness a healthy growth rate during the forecast period. The rising cancer rates, improved healthcare infrastructure, and growing numbers of medical device companies developing treatment products are major factors driving the regional market growth. Furthermore, the increasing awareness about healthcare services and the growing government initiatives are further propelling the market growth in this region. The Latin American, Middle Eastern, and African regions will grow moderately during the forecast period.
Have Queries? Speak to an expert, or To Download/Request a Sample, Click here.
Scope of the Report
| Report Attribute | Details |
| Market Value (2022) | USD 2,452 Million |
| Market Size (2032) | USD 4,786 Million |
| CAGR (from 2022 to 2032) | 7.1% |
| North America Revenue Share | 43% |
| Europe Revenue Share | 25% |
| Historic Period | 2016 to 2022 |
| Base Year | 2022 |
| Forecast Year | 2023 to 2032 |
Market Drivers
The global companion diagnostics market is driven by several factors, including the increasing number of cancer cases has increased the demand for CDx assays that are accurate and convenient. The Key manufacturers are focusing on developing new tests that evaluate multiple tumor biomarkers to determine specific molecular profiles for a patient with cancer and increase the Market for companion diagnostics. The increasing demand for quality healthcare services and the need for cost-effective solutions drive the growth of the global companion diagnostics market.
Market Restraints
On the other hand, the Market is expected to face challenges from a lack of regulatory framework in determining sensitivity and specificity while also making therapeutic decisions. In vitro diagnostic medical device regulation (IVDR) is being implemented by the European Union. The new law introduces a broad range of strict requirements regarding scientific validity and clinical performance. The critical factor in adopting devices is the availability of suitable reimbursement policies and regulations. The lack of reimbursement policies and strict rules will hinder global companion diagnostics market growth over the forecast period (2023-2032).
Market Opportunities
In the companion diagnostics market, there is high competition among the key market players. Just another organization can maintain the high-capital expenses and the significantly increased cost of Research & development and production. This will keep the new market players from entering the global Market. Companies in the companion diagnostics market also focus on providing better customer service and improving the quality of healthcare services.
Grow your profit margin with Market.us - Purchase Companion Diagnostics Market Premium Report at https://market.us/purchase-report/?report_id=57777
Report Segmentation of the Global Companion Diagnostics Market
By Product and Service Insight
The global companion diagnostics market is derived from products and services such as S assays, kits and reagents, software, and services. Assays are used to analyze a substance to determine its quality or composition. A kit is a collection or set of equipment, materials, or instructions for a specific purpose. The last segment, a reagent, is a chemical used in laboratory testing to make or measure other compounds. The above segment propels the global companion diagnostics market.
By Technology Insight
The global companion diagnostics market is segmented by technology analysis, polymerase chain reaction, next-generation sequencing, in situ hybridization, and immunohistochemistry. The polymerase chain reaction segment dominates the global companion diagnostics market through the launch of new products & regulatory approvals.
By Indication Insight
The global companion diagnostics market can be divided into lung cancer, breast cancer, colorectal cancer, leukemia, and melanoma. In 2022, the market share for companion diagnostics will be the highest in the malignant cancers section. Many factors contribute to the growth of the global companion diagnostics market, such as key players focusing on innovation to create advanced tests for cancer drugs and concentrating on developing innovative technology. The number of biomarker tests for targeted cancer drugs is also increasing.
By End-User Insight
The global companion diagnostics market is segmented by End-User analysis is pharmaceutical and biopharmaceutical companies, reference laboratories, and contract research organizations. The pharmaceutical & biopharmaceutical company End-User segment is expected to grow at the highest rate of CAGR over the forecast period (2023-2032) due to worldwide product launches and recent technological advances.
For more insights on the historical and Forecast global companion diagnostics market data from 2016 to 2032 - download a sample report at https://market.us/report/companion-diagnostics-market/request-sample/
Market Segmentation
By Product and Service
- S Assays
- Kits and Reagents
- Software and Services
By Technology
- Polymerase Chain Reaction
- Next Generation Sequencing
- In Situ Hybridization
- Immunohistochemistry
By Indication
- Lung Cancer
- Breast Cancer
- Colorectal Cancer
- Leukemia
- Melanoma
By End-User
- Pharmaceutical and Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
By Geography
- North America
- The US
- Canada
- Mexico
- Western Europe
- Germany
- France
- The UK
- Spain
- Italy
- Portugal
- Ireland
- Austria
- Switzerland
- Benelux
- Nordic
- Rest of Western Europe
- Eastern Europe
- Russia
- Poland
- The Czech Republic
- Greece
- Rest of Eastern Europe
- APAC
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- Indonesia
- Malaysia
- Philippines
- Singapore
- Thailand
- Vietnam
- Rest of APAC
- Latin America
- Brazil
- Colombia
- Chile
- Argentina
- Costa Rica
- Rest of Latin America
- Middle East & Africa
- Algeria
- Egypt
- Israel
- Kuwait
- Nigeria
- Saudi Arabia
- South Africa
- Turkey
- United Arab Emirates
- Rest of MEA
Competitive Landscape
The competitive landscape of the Market has also been examined in this report. Some of the major players include
- Abbott (U.S.)
- Agilent Technologies, Inc. (U.S.)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Guardant Health (U.S.)
- QIAGEN (Germany)
- Myriad Genetics, Inc. (U.S.)
- Illumina, Inc. (U.S.)
- Thermo Fisher Scientific Inc. (U.S.)
- BIOMERIEUX (France)
- Myriad Genetics, Inc. (U.S.)
- Other Key Players.
Recent Development of the Global Companion Diagnostics Market
- In August 2021: QIAGEN N.V. and OncXerna Therapeutics Inc. developed Next-Generation Sequencing (NGS), which is a companion diagnostic to OncXernas’s product candidate, Navicixizumab, which is a product candidate for treating patients with ovarian carcinoma.
- In October 2022: F. Hoffmann-La Roche Ltd. granted PATHWAY. This companion diagnostic helps to identify specific patients with HER2 metastatic breast cancer who have been approved for ENHERTU (HER2-directed anti-drug conjugate) treatment.
- In October 2022: HMNC Brain Health raised US$ 14.2 million in funding to support precision psychiatry programs. These programs combine pharmaceuticals and companion diagnostics to identify patient groups that will benefit from the treatments.
- In June 2022: OmniSeq Corporation announced the launch of OmniSeq INSTsm. This tissue-based test incorporates next-generation sequencing (NGS). This test will improve precision oncology and patient outcomes.
Browse More Related Reports:
- In Vitro Diagnostics (IVD) Market size is expected to be worth around USD 149.4 Bn by 2032 from USD 95.3 Bn in 2022, growing at a CAGR of 4.7%.
- Point-Of-Care Diagnostics Market size is expected to be worth around USD 85.8 million by 2032 from USD 40.9 million in 2022, growing at a CAGR of 7.95%.
- Cancer Diagnostics Market size is expected to be worth around USD 332.4 Billion by 2032 from USD 171.6 Billion in 2022, growing at a CAGR of 7%.
- Blood Transfusion Diagnostics Market Market size is expected to be worth around USD 9,095 Mn by 2032 from USD 4,702 Mn in 2022, growing at a CAGR of 7%.
- Tuberculosis Diagnostics Market was valued at USD 2.9 Bn in 2022 and is projected to reach USD 5.80 Bn by 2032, at a CAGR of 6.5%
About Us:
Market.US (Powered by Prudour Pvt Ltd) specializes in in-depth market research and analysis and has been proving its mettle as a consulting and customized market research company, apart from being a much sought-after syndicated market research report-providing firm. Market.US provides customization to suit any specific or unique requirement and tailor-makes reports as per request. We go beyond boundaries to take analytics, analysis, study, and outlook to newer heights and broader horizons.
Follow Us on LinkedIn | Facebook | Twitter
Our Blog:
