TUCSON, Ariz., Aug. 26, 2014 (GLOBE NEWSWIRE) -- SynCardia Systems, Inc., manufacturer of the SynCardia temporary Total Artificial Heart, is high on the Inc. 5000 named by the business magazine Inc.
"SynCardia manufactures the world's only FDA, Health Canada and CE approved Total Artificial Heart, which is used as a bridge to a matching donor heart transplant," says Michael P. Garippa, SynCardia's CEO and President. "It has become the standard of care for patients dying from end-stage biventricular heart failure."
This year the business magazine ranked SynCardia 137th among 377 companies in the health industry and number 1,698 on the list of 5,000 companies. The privately held Tucson-based firm also was ranked 34th among 104 fastest growing companies in Arizona.
The 2014 rankings put SynCardia around the top third in the list.
This is the fourth time SynCardia has made the Inc. 5000 list. It rose from a ranking of 3,136th place in 2011, showing a 46% improvement in this year's ranking.
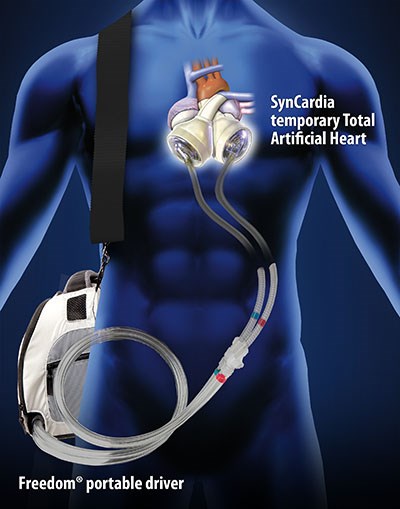
The company also manufactures the Freedom® portable driver, which powers the SynCardia Total Artificial Heart and allows clinically stable patients to be discharged from the hospital to wait for matching donor hearts at home and in their community. Patients, hospitals and insurance companies can save thousands of dollars of in-hospital costs for this portion of patient care.
›› See Multimedia Release
For additional information, please visit: http://www.syncardia.com/
- Like SynCardia on Facebook
- Follow SynCardia on Twitter – @SynCardia
- Connect with SynCardia on LinkedIn
- Share and Discover on Google+
About the SynCardia temporary Total Artificial Heart
SynCardia Systems, Inc. in Tucson, Arizona is the privately-held owner and manufacturer of the world's first and only FDA, Health Canada and CE approved Total Artificial Heart.
Originally used as a permanent replacement heart, the SynCardia Total Artificial Heart is currently approved as a bridge to transplant for people suffering from end-stage biventricular heart failure in which both ventricles can no longer pump enough blood for a person to survive.
More than 1,350 implants of the SynCardia Total Artificial Heart accounts for over 400 patient years of life on the device. Since January 2011 more than 400 SynCardia Hearts have been implanted.
SynCardia Systems also manufactures the Freedom® portable driver, which powers the SynCardia Heart while allowing clinically stable patients to leave the hospital to live at home and in their communities. The wearable Freedom driver has been used by over 175 patients, accounting for over 100 years of support.
A photo accompanying this release is available at http://www.globenewswire.com/newsroom/prs/?pkgid=27305