— FDA clears new device for use along with a standard colonoscope to improve visibility in areas behind folds in colon wall —
SUNNYVALE, Calif., Nov. 19, 2014 (GLOBE NEWSWIRE) -- Avantis Medical Systems, Inc., a technology leader in developing novel digital imaging devices, today announced that its Third Eye® Panoramic™ device for colonoscopy has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
Photos accompanying this release are available at
http://www.globenewswire.com/newsroom/prs/?pkgid=28530
http://www.globenewswire.com/newsroom/prs/?pkgid=28531
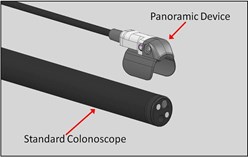
The Third Eye Panoramic device utilizes technology that was developed for the company's earlier product, the Third Eye® Retroscope®. Like its predecessor, the Panoramic device is designed to help physicians see behind folds in the wall of the colon that can hide pre-cancerous polyps called adenomas, but with improved ease-of-use and reduced expense.
While the Retroscope device had one additional camera that was aimed backward, the new Panoramic device instead has two video cameras that are directed laterally from its left and right sides. The video monitor displays the lateral images on both sides of the colonoscope's forward image, resulting in an ultra-wide-angle view of more than 300 degrees. This "panoramic" view reveals areas behind folds and flexures (sharp turns) in the colon.
The Third Eye Panoramic device is attached to the tip of the colonoscope at the beginning of the procedure, and it can be used during both the insertion and withdrawal phases. Because it clips onto the outside of the colonoscope, it leaves the channel completely free, in contrast to the earlier device that occupied the channel of the colonoscope and had to be removed when the physician inserted instruments to remove polyps.
"We spent thousands of hours observing endoscopists as they performed colonoscopies using the Third Eye Retroscope, and we listened to their concerns regarding the ease of use," said Jack Higgins, MD, Chief Medical Officer for Avantis Medical Systems. "They liked the fact that additional cameras allowed them to see more of the colon, and they asked us to find a way to do that more easily and inexpensively, and without occupying the channels of their colonoscopes."
Dr. Sang Kim and Dr. Moshe Rubin recently performed a feasibility study of the Third Eye Panoramic device at New York Hospital Queens Weill Cornell Medical College.1 They found that the Panoramic device worked well in conjunction with a standard colonoscope and provided enhanced, wide-angle imaging of the colon without adverse events. The results of the second phase of that study demonstrated an adenoma detection rate of 48% and were presented at the American College of Gastroenterology Annual Scientific Meeting (ACG 2014) in Philadelphia on October 21.
"Improving the quality of colonoscopy with enhanced imaging has been shown in recent studies to increase our ability to detect precancerous polyps. The elegant approach offered by Avantis Medical in developing the Third Eye Panoramic cap offers significant advantages. Gastroenterologists can continue to utilize their existing high definition standard colonoscopes without new investments in capital equipment," said Dr. Rubin. "Additionally, the non-obtrusive video cap does not change the dynamics of scope insertion or maneuverability, thus providing an enhanced nearly 360-degree view without sacrificing scope familiarity, usability or efficiency."
The Third Eye Panoramic device is designed to be used along with any standard adult or pediatric size colonoscope, so it avoids any substantial capital expense and it allows physicians to continue using the high-quality technologies in which their facilities have already invested, generally including colonoscopes with high-resolution or even high-definition video cameras.
"Now that the Third Eye Panoramic device has been cleared by the FDA for marketing, it will allow other leaders in the field of gastroenterology to provide us with feedback as we develop a resposable version of the device," said Doug Gielow, Avantis' Vice President for Sales & Marketing. "At the same time we can finalize our selection of a partner with broad domestic and international commercial distribution that is beyond the reach of a development company."
About Colorectal Cancer
Colorectal cancer (CRC) is the second-leading cause of cancer-related deaths in the United States. According to the American Cancer Society, about 150,000 people in the U.S. are diagnosed with CRC each year and almost 50,000 die from it. Screening and surveillance colonoscopies allow CRC to be found earlier, when the disease is easier to cure, and cancers can be prevented if adenomas are removed before they become malignant.
Colonoscopy is the "gold standard" for detecting cancers and pre-cancerous adenomas in the colon. However, extensive research has shown that even when performed carefully by experienced physicians, standard colonoscopy misses approximately 24% of adenomas of all sizes.2-4 Even more importantly, standard colonoscopy misses 12% of large adenomas – measuring at least 1 cm in diameter – which are the ones most likely to progress to colon cancer.5-7
A number of factors have been shown to affect adenoma detection rates, including adequacy of bowel preparation and the amount of time spent examining the lining of the colon. However, approximately 2/3 of adenomas that are missed are located behind the numerous folds in the colon, where they are hidden from the forward view of the colonoscope.5 Adenomas and other abnormalities in those "blind spots" behind folds are easily missed with a standard colonoscope.
In fact, over 8% of all cases of CRC are "interval cancers" – found within 2-3 years after the patient has had a "normal" colonoscopy.8 Previous research had shown that patients examined by endoscopists with higher adenoma detection rates (ADR) have a lower likelihood of developing an interval cancer. Now a recent study in the New England Journal of Medicine has reported that for each 1% increase in an endoscopist's ADR, there is a 3% decrease in the risk for interval cancer and a 5% decrease in the risk for fatal interval cancer.8
About Avantis Medical Systems and the Third Eye Panoramic device
Avantis Medical Systems is focused on delivering cost-effective solutions for improved detection and prevention of cancers of the gastrointestinal tract. The company has an extensive portfolio of patents covering innovative devices based on the convergent technologies of micro-chips and enhanced video processing systems.
The Third Eye Panoramic device utilizes ground-breaking technology that was developed for the company's previous device, the Third Eye Retroscope, which was shown in clinical studies to help physicians find up to 23-25% more pre-cancerous adenomas than a standard colonoscope alone.
The Third Eye Panoramic device features two video cameras that are directed laterally. Combining their images with the one from the colonoscope's forward-viewing camera creates an ultra-wide-angle panoramic view.
Lazar Partners for Avantis Medical Systems, Inc.
Chantal Beaudry
(646) 871-8480 / (917) 494-0227
cbeaudry@lazarpartners.com
Avantis Medical Systems, Inc.
Doug Gielow
(408) 728-5126
dgielow@avantismedical.com
1. Rubin M, Bose KP, Kim SH. Successful Deployment and Use of Third Eye Panoramic, a Novel Side Viewing Video Cap Fitted on a Standard Colonoscope. Gastrointest Endosc 2014;79:AB466.
2. Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24-8.
3. Van Rijn JC, Reitsma JB, Dekker E, et al. Polyp Miss Rate Determined by Tandem Colonoscopy: A Systemic Review. Am J Gastroenterol 2006;101:343-50.
4. Heresbach D, Barrioz T, Ponchon T, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40:284-90.
5. Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Annals of Internal Medicine 2004;141:352-60.
6. Siersema PD, Rastogi A, DeMarco DC, et al. Retrograde-viewing device improves adenoma detection rate in colonoscopies for surveillance and diagnostic workup. World J Gastroenterol 2012;18:3400-8.
7. Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: an observational study. Gastrointest Endosc 2011;74:246-52.
8. Corley DA, Jensen CD, Mark AR, et al. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N Eng J Med 2014;370:1298-306.