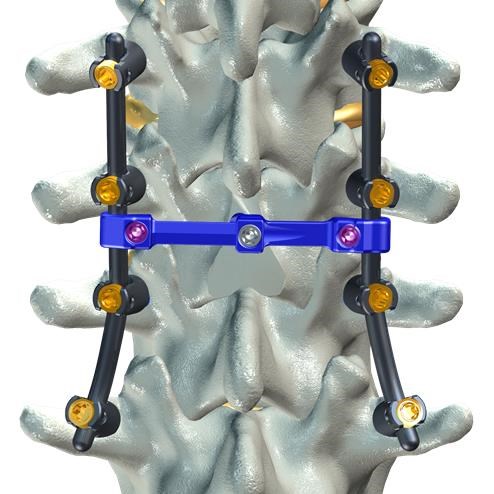
FLORENCE, S.C., Aug. 5, 2016 (GLOBE NEWSWIRE) -- DeGen Medical has received clearance from the FDA for its CONNECT-L™ Transverse Connector to be used with F1 MPS™ Modular Pedicle Screw System. The F1 MPS™ system is intended to provide immobilization and stabilization of posterior non-cervical spine.
The CONNECT-L Transverse Connector is small, low profile, and easy to use. The CONNECT-L™ rod-to-rod connection provides improved construct stability and rigid fixation to the posterior spinal system. The CONNECT-L™ includes fixed and variable sizes for posterior thoraco-lumbar-sacral spine surgeries. The variable option offers 6 degrees of freedom, allowing additional maneuverability for complex spinal stabilization procedures.
About DeGen Medical, Inc.
DeGen Medical, Inc. is a medical device development company dedicated to providing surgeons with innovative products engineered to improve quality of life for patients with complex spinal disorders. World-class implants, coupled with intuitively-designed instrumentation, provide a complete package to promote superior surgical outcomes. Our passion to advance spine-care solutions is driven by clinical insights, sound research, and science-based design. DeGen Medical maintains the highest quality standards to provide reliable products and safeguard patient health.
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=41030