

Rodolphe has over 20 years’ experience in the pharma and biotech industry. Rodolphe joined Coave Therapeutics in 2020 from Enterome, which he co-founded. During his tenure at Enterome, Rodolphe executed over 15 transactions, including major industrial partnerships with Takeda, J&J, BMS, Abbvie and Nestle Health Sciences, generating over €100 million in upfront, R&D payments, and equity investments. He was also actively involved in multiple fundraising rounds. Rodolphe has previously worked in business development at TcLand Expression and Genzyme, and as a sell-side equity analyst at Natixis Bank.
Rodolphe graduated with a M.Sc. degree in Biochemical Engineering from Polytech Marseille and is a Certified European Financial Analyst from EFFAS-SFAF.

Patricia has over 20 years’ experience in the biotech industry leading R&D, preclinical and clinical operations as well as manufacturing for the accelerated development of innovative biologics, including advanced cell & gene therapies. Patricia joined Coave from Skinosive where she served as Chief Operating/Technology Officer until November 2021. At Skinosive she managed all operational aspects of the business, proactively driving the company towards achieving its development goals.
Patricia obtained her PhD in Molecular and Cellular Biology from Paris VI University and completed her postdoctoral research at McGill University.

Lolita is a genetic medicine expert with over a decade of pharmaceutical and biotech experience in gene therapy. Her expertise ranges from the development of novel target concepts to the clinical advancement of candidates. She joined Coave Therapeutics from Janssen Pharmaceuticals (J&J), where she managed the Translational Gene Therapy Research Team responsible for the characterization and selection of gene therapy candidates in multiple therapeutic areas. Alongside this, Lolita led the development and strategic implementation of novel delivery and immunomodulation platforms for ocular gene therapy applications. Prior to J&J, she worked at Spark Therapeutics (part of the Roche Group) where she managed the Ocular Platform Team and oversaw the continuous optimization of innovative gene therapy vectors and novel gene therapy strategies.
Lolita holds a PhD in Biotechnologies and Therapeutics from the University of Nantes (France).

Gaelle joined Coave Therapeutics in 2019 as a project manager. She holds a Ph.D. in Molecular Oncology from Paris University and spent over 20 years as a research scientist in renowned academic institutions in France (Institut Pasteur), Canada (McGill University) and the US (NIH, University of Michigan). In 2014, she transitioned to project management and coordinated several large, collaborative translational research studies on rare genetic sensory disorders of the eye and ear and the development of AAV-mediated gene therapies for these indications. She brings her unique expertise to the global management of all the non-clinical projects at Coave Therapeutics.

Julien has almost 20 years of global, regional and local healthcare legal affairs experience among corporate, commercial, medical, R&D, clinical operations and M&A activities. Julien joined Coave in May 2022 from Galapagos where he served as Senior Legal Counsel Director providing legal support and advice to its global teams.
Prior to Galapagos, Julien spent over 15 years in the legal team at Genzyme, most recently as Legal Director at Sanofi Genzyme following its acquisition. Julien developed and led the legal department for Genzyme’s French entity, supporting the growing business in five therapeutic areas, managing the launch of products in addition to supporting Genzyme’s acquisition by Sanofi.
Julien is a Business Law graduate from the universities of Lyon and Strasbourg.

Cédric brings 15 years of experience in financial auditing, management, and transaction consulting. Prior to joining Coave Therapeutics, he was at EY in Paris, where he specialized in private equity and growth companies and acquired deep expertise in financial auditing and transaction consulting. Cédric is an engineer who graduated from the Grande École d’Ingénieurs (Institut supérieur d’électronique de Paris – ISEP) in Paris and holds a degree in corporate finance from the EM Lyon Business School.
Frederic currently serves as the President and Chief Executive Officer of LogicBio (Nasdaq: LOGC), a clinical stage genetic medicines company pioneering gene editing and delivery platforms to address rare and serious disease. He has more than 25 years of executive leadership and industry experience in biotechnology, pharmaceuticals, and medical devices.
Prior to LogicBio, Frederic has worked at Genzyme, Pervasis Therapeutics, Shire and aTyr Pharma as General Manager, President & CEO, Senior Vice President and COO respectively. Frederic holds a BSc in Physics, an MSc in Management and completed his MBA at INSEAD, Fontainebleau, France.
Dr. Danos has over 30 years’ experience in gene therapy, as a scientist pioneering gene transfer technologies and as a leader in the biotech and pharmaceutical industry. He currently serves as the Chief Scientific Officer (CSO) of RegenxBio (Nasdaq: RGNX), a clinical stage biotechnology company developing a proprietary adeno-associated virus (AAV) gene delivery platform for the treatment of retinal, neurodegenerative and metabolic diseases.
Prior to RegenxBio, Olivier has undertaken a number of clinical research and executive leadership roles including Senior Vice President at Biogen and Kadmon Pharmaceuticals, Director of the Gene Therapy Consortium at UCL, CSO at Genethon, as well as Director of Research at the CNRS (Centre national de la recherche scientifique – The French National Centre for Scientific Research) and Principal Investigator at Institut Pasteur. Olivier completed his PhD at the University Paris Diderot.
Claudia is a scientist and entrepreneur with over 20 years’ research and industry experience. She most recently served as Senior Vice President of Portfolio Strategy at Astellas Pharma in Tokyo, Japan. Prior to this, Claudia co-founded Universal Cells, where she served as CEO until it was acquired by Astellas for over $100 million. Claudia also co-founded Halo-Bio RNAi Therapeutics and served as its Chief Scientific Officer.
Claudia holds a PhD in Molecular Biology from the University of Paris and an MBA in International Management from Ecole des Ponts Business School, Paris, France. She was awarded the 2018 EY Entrepreneur of the Year award in Life Sciences for the Pacific Northwest Region, USA.
Bruno is a Partner at Seroba Life Sciences. He has a background in venture capital and investment banking, with a focus on the pharmaceutical, biotechnology and medical device industries.
Bruno graduated in 1998 with a PharmD, from the Université René Descartes, Paris V and in the same year completed a master’s degree in Strategic Management at HEC.
Prior to joining the firm in 2017, Bruno was a Partner at Omnes Capital (Paris), in charge of life sciences investments for the venture capital team. His previous venture capital experience was at Atlas Venture (Paris/London) and CDP Capital (Paris/Montreal). He started his career in 1999 in London, in the healthcare teams of the investment banking divisions of Deutsche Bank and later Merrill Lynch. Bruno brings a wealth of experience and strong networks, particularly in continental Europe, where he is based.
Rodolphe has over 20 years’ experience in the pharma and biotech industry. Rodolphe joined Coave Therapeutics in 2020 from Enterome which he co-founded. During his tenure at Enterome, Rodolphe executed over 15 transactions, including major industrial partnerships with Takeda, J&J, BMS, Abbvie and Nestle Health Sciences, generating over €100 million in upfront, R&D payments, and equity investments. He was also actively involved in multiple fundraising rounds. Rodolphe has previously worked in business development at TcLand Expression and Genzyme, and as a sell-side equity analyst at Natixis Bank.
Rodolphe graduated with a degree in Biochemical Engineering from Polytech Marseille and is a Certified European Financial Analyst from EFFAS-SFAF.
Emmanuelle is Partner in the Seed Investment team at Novo Holdings, with over 16 years’ experience as a life science venture investor. Prior to Novo Holdings, Emmanuelle was Partner at Auriga Partners and oversaw the Seed Stage Investment Programme at Omnes Capital (formerly Crédit Agricole Private Equity). Emmanuelle has a PhD in Microbiology from the Universite Paris Cite and an MSM, Medical management from ESCP Business School.
Current positions: Member of the Board of Directors of Minervax, Draupnir, Corwave, Heparegenix, and BiOrigin.
Jean-François Morin is an Investment Director at Bpifrance with over a decade of experience in investment and finance. He specializes in life sciences, driving strategic investments in biotech and medtech companies. With a strong background in finance and venture capital, he has successfully managed and structured numerous investment deals. Jean-François currently serves on the Boards of ONA Therapeutics, Egle therapeutics, Orphalan, May Health and Pixee Medical. He began his carreer at MAZARS as a Financial Auditor, followed by a role at CDC Enterprises. He hold a Master’s degree in Finance from EDHEC Business School.
Dr. Danos has over 30 years’ experience in gene therapy, as a scientist pioneering gene transfer technologies and as a leader in the biotech and pharmaceutical industry. He currently serves as the Chief Scientific Officer (CSO) of RegenxBio (Nasdaq: RGNX), a clinical stage biotechnology company developing a proprietary adeno-associated virus (AAV) gene delivery platform for the treatment of retinal, neurodegenerative and metabolic diseases.
Prior to RegenxBio, Olivier has undertaken a number of clinical research and executive leadership roles including Senior Vice President at Biogen and Kadmon Pharmaceuticals, Director of the Gene Therapy Consortium at UCL, CSO at Genethon, as well as Director of Research at the CNRS (Centre national de la recherche scientifique – The French National Centre for Scientific Research) and Principal Investigator at Institut Pasteur. Olivier completed his PhD at the University Paris Diderot.
Alain Wagner, PhD is currently Director of the Laboratory of Functional Chemosystems at the University of Strasbourg with a wealth of industry experience. Alain contributes to cutting-edge research concentrated on innovation and industry value creation. His experience in the up-and-coming field of controlling exogenous chemistry in living organisms promises major advances in many therapeutic, diagnostic and technology applications, including treatment for neural diseases. Dr Wagner has published more than 150 articles in leading peer-reviewed scientific journals and is the inventor of 25 patents.
Anne des Rieux, PhD is a Professor at UCLouvain, Belgium. She is one of the PI leading the Advanced Drug Delivery and Biomaterials unit at the Louvain Drug Research Institute. She develops innovative drug and stem cell delivery systems for unmet neurological needs such as spinal cord injury and multiple sclerosis.
Aravind Asokan, PhD is a specialist in synthetic virology, blending the fields of protein engineering, RNA biology and virology to enable gene editing and gene therapy platforms. He currently serves as Professor and Director of Gene Therapy at Duke University School of Medicine and his lab has pioneered several unique platforms that are being developed into life-changing medicines.
Juliette Hordeaux, DVM, PhD has over a decade of experience in the translational AAV gene therapy field and currently holds the position of Executive Director of translational research at the Gene Therapy Program,University of Pennsylvania. In this role, she leads a team of scientists investigating AAV therapies for rare monogenic disorders, with specific interest in AAV-mediated toxicity and ways to develop safer gene therapy modalities. Juliette has successfully guided five AAV-programs through Investigational New Drug (IND) applications and is regularly invited to speak at events discussing AAV-mediated toxicity.
Mark Kay, MD, PhD is respected worldwide for his work in gene therapy and has been responsible for the coordination of many national and international gene therapy conferences and organizations, including the first Gordon Conference related to gene therapy. Previously, Mark was one of the founders of the American Society of Gene Therapy and served as its president in 2005-2006.
Robert Kotin, PhD has been a leader in AAV research for almost 40 years including 20 years at the National Institutes of Health where he led the Laboratory of Molecular Virology and Gene Therapy. His research on AAV molecular biology has led to novel AAV vectors for somatic cell gene therapy. Since 2016, he has served as an Adjunct Professor at UMass Medical School, with research focused on the discovery of novel gene therapy vectors. He is the Scientific Co-Founder and Chief Science Advisor of Carbon Biosciences, that is developing novel parvovirus gene therapy vectors.


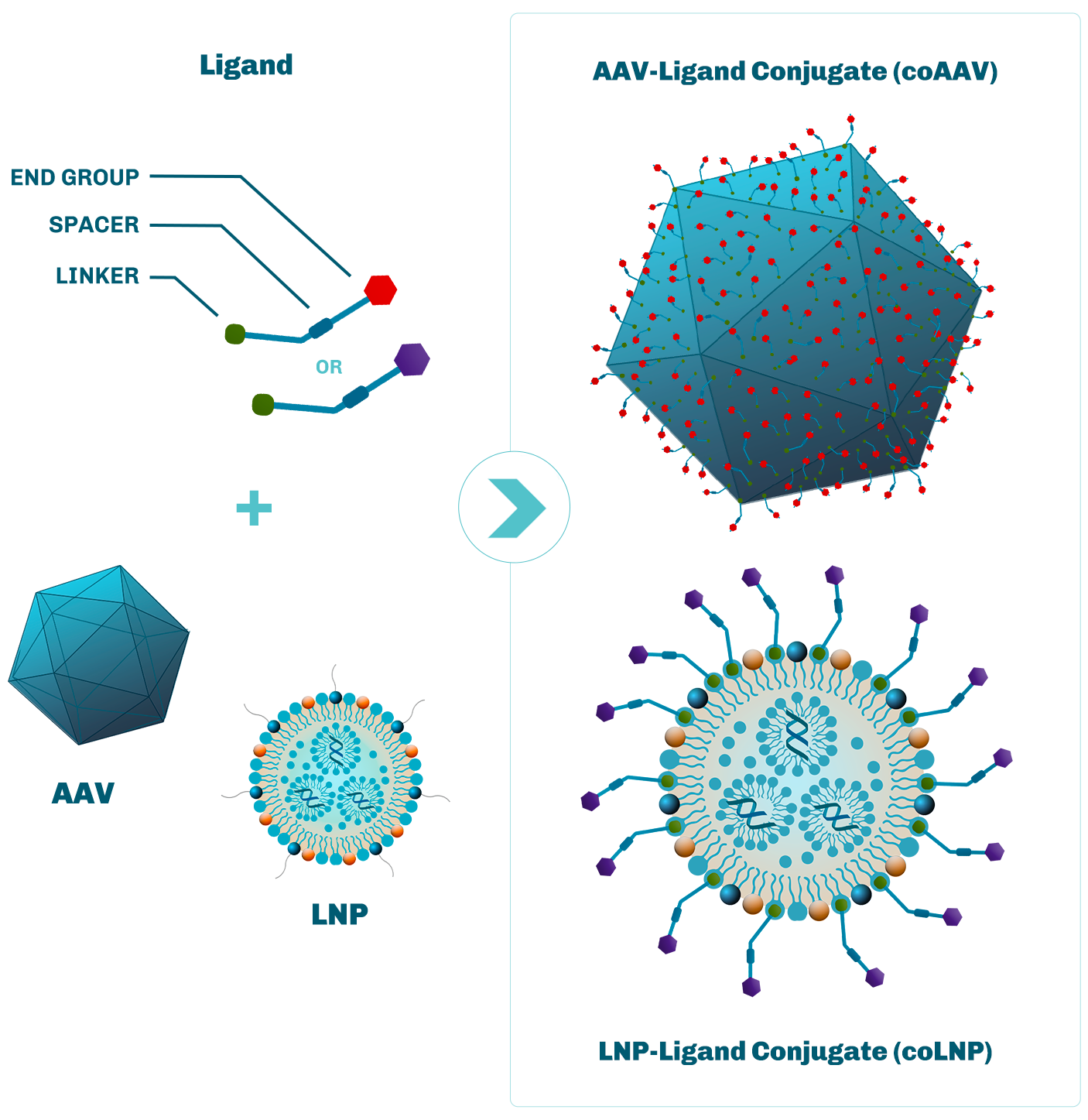
Site-specific conjugation onto capsid proteins enables altering of extracellular capsid sequestration, by blocking the binding of the AAV capsid to extracellular motifs.

Ligands are rationally designed to improve cell and tissue targeting, based on defined ligand-receptor interactions.

Site-specific conjugation of capsid proteins leads to improvement of intracellular AAV capsid trafficking and payload delivery to the nucleus.
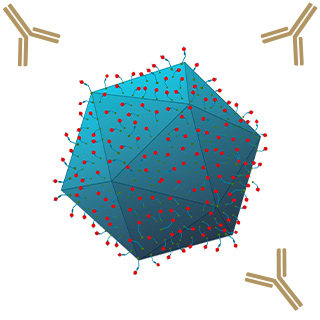
Ligand conjugation reduces coAAV exposure to immune reaction and neutralizing antibodies.
The misfolding and accumulation of disease-related proteins are common hallmarks of several neurodegenerative diseases. As examples, beta-amyloid and tau in Alzheimer’s disease (AD), alpha-synuclein (aSyn) in Parkinson disease (PD) and related disorders, mutated huntingtin in Huntington’s disease (HD), and TAR DNA-binding protein 43 (TDP-43) in Amyotrophic Lateral Sclerosis (ALS), are shown to contribute to neurodegeneration and disease progression.
The presence of TDP-43 proteinopathy, characterized by the accumulation of highly modified and misfolded TDP-43 molecules in the cytoplasm, is a hallmark of various neurodegenerative diseases including ALS. Proposed mechanisms underlying TDP-43 proteinopathies involve disruptions in nuclear-cytoplasmic localization homeostasis, aggregation of ubiquitinated and hyper-phosphorylated TDP-43, and increased protein truncation of cytoplasmic TDP-43.
Pathological accumulation of aSyn is the common distinguishing trait amongst the group of brain disorders known as synucleinopathies, which include PD, Dementia with Lewy bodies (DLB), and Multiple System Atrophy (MSA). These disorders progressively develop neuronal and glial inclusions enriched with misfolded, phosphorylated and insoluble aSyn.
Over the past decade, several treatment strategies directly targeting protein aggregates have been evaluated in preclinical and clinical studies. For example, in ALS, several therapeutic strategies, spanning biologics to small molecules, that directly address TDP-43 pathology are under evaluation. In PD, diverse approaches include removal of aggregated aSyn with passive or active immunization or by expression of vectorized antibodies, modulating kinetics of misfolding with small molecule anti-aggregants, lowering aSyn gene expression by antisense oligonucleotides or inhibitory RNA.
The autophagy lysosomal pathway (ALP) is a central cellular pathway enabling the degradation of toxic protein aggregates. Key factors of the ALP, such as Glucocerebrosidase (GBA1) and Transcription Factor EB (TFEB), have been shown to play important roles in protein aggregate clearance mechanisms (Figure X).
At Coave, using our ALIGATER™ platform, we have generated a coAAV-based genetic medicine approach to deliver safe, low doses of GBA1 and TFEB precisely targeted to relevant structures (tissues and cells) of the central nervous system (CNS), aiming to activate or restore the autophagy and lysosomal functions to eliminate or prevent the accumulation of toxic protein aggregates associated with neurodegenerative disease.
The misfolding and accumulation of disease-related proteins are common hallmarks of several neurodegenerative diseases. As examples, beta-amyloid and tau in Alzheimer’s disease (AD), alpha-synuclein (aSyn) in Parkinson disease (PD) and related disorders, mutated huntingtin in Huntington’s disease (HD), and TAR DNA-binding protein 43 (TDP-43) in Amyotrophic Lateral Sclerosis (ALS), are shown to contribute to neurodegeneration and disease progression.
The presence of TDP-43 proteinopathy, characterized by the accumulation of highly modified and misfolded TDP-43 molecules in the cytoplasm, is a hallmark of various neurodegenerative diseases including ALS. Proposed mechanisms underlying TDP-43 proteinopathies involve disruptions in nuclear-cytoplasmic localization homeostasis, aggregation of ubiquitinated and hyper-phosphorylated TDP-43, and increased protein truncation of cytoplasmic TDP-43.
Pathological accumulation of aSyn is the common distinguishing trait amongst the group of brain disorders known as synucleinopathies, which include PD, Dementia with Lewy bodies (DLB), and Multiple System Atrophy (MSA). These disorders progressively develop neuronal and glial inclusions enriched with misfolded, phosphorylated and insoluble aSyn.
Over the past decade, several treatment strategies directly targeting protein aggregates have been evaluated in preclinical and clinical studies. For example, in ALS, several therapeutic strategies, spanning biologics to small molecules, that directly address TDP-43 pathology are under evaluation. In PD, diverse approaches include removal of aggregated aSyn with passive or active immunization or by expression of vectorized antibodies, modulating kinetics of misfolding with small molecule anti-aggregants, lowering aSyn gene expression by antisense oligonucleotides or inhibitory RNA.
The autophagy lysosomal pathway (ALP) is a central cellular pathway enabling the degradation of toxic protein aggregates. Key factors of the ALP, such as Glucocerebrosidase (GBA1) and Transcription Factor EB (TFEB), have been shown to play important roles in protein aggregate clearance mechanisms (Figure X).
At Coave, using our ALIGATER™ platform, we have generated a coAAV-based genetic medicine approach to deliver safe, low doses of GBA1 and TFEB precisely targeted to relevant structures (tissues and cells) of the central nervous system (CNS), aiming to activate or restore the autophagy and lysosomal functions to eliminate or prevent the accumulation of toxic protein aggregates associated with neurodegenerative disease.
ALS is a rare neurodegenerative condition marked by the swift and relentless decline of motor neuron function and survival, resulting in paralysis and ultimately respiratory failure, culminating in death usually within five years after diagnosis. The etiology of ALS remains unknown and non-genetically defined in 90-95% of cases, with no cure .
The incidence of ALS in the US and Europe is approximately 2 in 100,000, equating to approx. 30,000 patients in the US and 50,000 in Europe.
Protein aggregation and dysregulation of the autophagy-lysosome pathway are hallmarks of ALS, with evidence of accumulation of autophagosomes, disrupted lysosomes, and TDP-43 aggregates in patients’ brains (Beckers et al., 2021). Transcription factor EB (TFEB) has emerged as a master regulator of the autophagy lysosomal pathway, and is therefore a promising therapeutic target for clearing toxic aggregates (Napolitano and Ballabio, 2016). Stage-dependent alterations in TFEB expression have been observed in ALS models, with decreased TFEB activity in patient brain samples. TFEB overexpression mediated by gene therapy is anticipated to mitigate toxic protein accumulation thus offering a strategy to halt motor neuron degeneration and impede ALS progression. TFEB represents a crucial avenue for ALS treatment, echoing its significance in other neurodegenerative diseases.
Our gene therapy product candidate, CTx-TFEB, utilizes a coAAV vector to deliver a gene sequence encoding functional TFEB transcription factor. CTx-TFEB has been optimized for improved transduction and distribution of the TFEB gene in the key structures of the CNS involved in ALS.
Inherited retinal dystrophies are rare ophthalmic pathologies that can be divided into two groups:
Retinitis pigmentosa is the most common form of inherited retinal dystrophy representing 50% of all retinal dystrophies.
While multiple genes are implicated in each of these groups, within each patient or family, only one causative gene is involved.
January, 09 – 2025
December, 17 – 2024
October, 22 – 2024
September, 24 – 2024
September, 6 – 2024
May, 7 – 2024
April, 29 – 2024
April, 25 – 2024
March, 27 – 2024
February, 29 – 2024
October, 12 – 2023
September, 28 – 2023
September, 21 – 2023
May, 31 – 2023
May, 17 – 2023
May, 4 – 2023
March, 16 – 2023
October, 20 – 2022
September, 26 – 2022
September, 20 – 2022
September, 14 – 2022
September, 6 – 2022
June, 30 – 2022
June, 9 – 2022
May, 4 – 2022
April, 27 – 2022
April, 13 – 2022
November, 15 – 2021
October, 12 – 2021
September, 23 – 2021
September, 16 – 2021
July, 21 – 2021
JOB OPENINGS
We are a talented, passionate group of colleagues with a desire to translate innovative science into novel gene therapies for patients with neurodegenerative and ocular diseases and beyond.
We are committed to building a vibrant team combining deep expertise in AAV vector engineering and genetic construct design, innovative and advanced therapeutic product development, and manufacturing.
We are looking for more talented individuals to join our team.
Headquarters
63bis avenue Ledru Rolin
75012 Paris – France
Labs
INSTITUT DU CERVEAU ET DE LA MOELLE EPINIÈRE – ICM
Hôpital Pitié-Salpêtrière
47 bd de l’Hôpital
75013 Paris