TAMPA, FL--(Marketwired - Jun 4, 2014) - Tampa Medical Innovations, Inc. (TaMI) has been selected as one of the 10 semi-finalists in the MedTech Innovator program, which is part of the 22nd Annual Medical Device Conference. The Conference, scheduled for June 12, 2014 in San Francisco, attracts more than 600 people and offers a $100,000 prize for the winner.
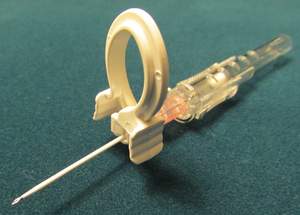
TaMI was selected based upon its lead product, the IV STAT-Clip™, which is expected to be commercially available by the end of 2014.
The STAT-Clip is a simple and affordable way to reduce bloodborne pathogen exposure due to IVC (intravenous catheterization) procedures. Antimicrobial resistance is spreading, resulting in an increase in the frequency and intensity of bloodborne diseases; the World Health Organization calls this the most serious problem facing modern medicine.
By preventing blood leakage during the initiation of an IV, the STAT-Clip can reduce blood pathogen exposure risks. Over one billion IVs are initiated every year, and about 35% of the time there is blood leakage requiring careful cleanup to minimize exposure risks.
The STAT-Clip was invented by Dr. Tariq Chaudhry of the Moffitt Cancer Center in Florida. "I consider the STAT-Clip to be a 'fail-safe' device, which anyone can use without reading an instruction manual, making prevention simple and routine in one of the most common medical procedures," says Chaudhry. "The STAT-Clip effectively prevents blood exposure during IVC placement and reduces the risk of hospital-acquired infections, an expensive but preventable complication, with a single-use clip that can be in every IVC kit worldwide."
The STAT-Clip human safety study will be completed in July of 2014. The preliminary trials with nurses are already generating excitement about a simple way to make routine IV placement safer for patients and for healthcare workers.
In 2011, at least 197 million IV procedures were performed in the United States alone. This market was valued at $4.69 billion in 2006 and its value was $5.65 billion in 2011, with a compound annual growth rate of 4.1%.
Bill Knab, acting CEO of TaMI, says, "This blood safety product has the potential to be ubiquitous worldwide, helping to protect people from bloodborne diseases that are increasingly difficult to treat. We look forward to partnering with global firms so that the benefits are available without delay. We will be able to go to sales within a few months of funding." The company has been funded to date by the founders and angel investors.
About TaMI
Tampa Medical Innovations, Inc. (TaMI) is a Medical Device company working on an innovative portfolio of products. TaMI has partnered with some of the leading clinical and academic institutions in the country to commercialize their "untapped" medical devices. The company's business model is to focus on simple Class I and II devices that are low cost, have large market potential and will monetize quickly for investors. Established in 2012, TaMI is a joint venture of Moffitt Cancer Center and Medical Device Development Group. www.tami-inc.com
About the 22nd Annual Medical Device Conference and MedTech Innovator
The MedTech Innovator competition is part of the 22nd Annual Medical Device Conference, which is hosted by law firm Wilson Sonsini Goodrich & Rosati (WSGR). A total of 267 companies submitted their plans, 10 were selected as semi-finalists, and the winner will be chosen following live presentations on June 12, 2014. RCT Ventures is sponsoring the $100,000 prize for the winner. http://medtechinnovator.com
Contact Information:
Company contact:
Scott Hampton
+1.678.468.8849