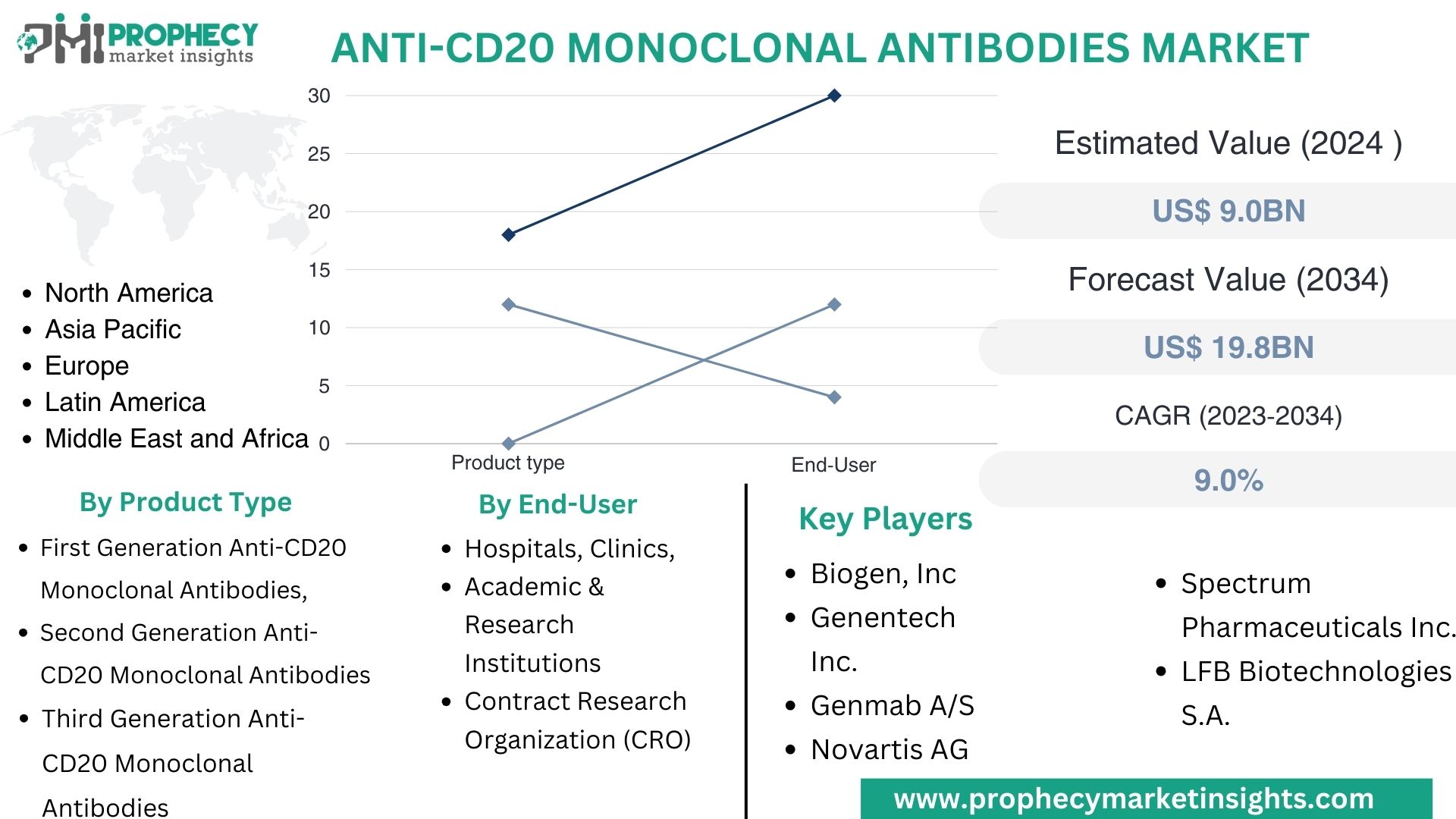
Covina, Feb. 01, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the Anti-CD20 Monoclonal Antibodies Market size was valued at about USD 9.0 Billion in 2024 and expected to grow at CAGR of 9.0% to extend a value of USD 19.8 Billion by 2034.”
What is Anti-CD20 Monoclonal Antibodies?
- Market Overview:
Anti-CD20 monoclonal antibodies are a class of therapeutic antibodies that target a specific protein called CD20 found on the surface of B cells, a type of white blood cell. These antibodies are designed to selectively bind to CD20, leading to the destruction or suppression of B cells. B cells play a crucial role in the immune system, and their abnormal activation or proliferation is associated with various autoimmune diseases and certain types of cancers, particularly B-cell lymphomas.
The main therapeutic applications of anti-CD20 monoclonal antibodies include:
- Autoimmune Diseases: These antibodies are used to treat autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis by modulating the immune response and reducing inflammation.
- Hematologic Cancers: Anti-CD20 monoclonal antibodies are widely employed in the treatment of B-cell lymphomas, including non-Hodgkin's lymphoma and certain types of leukemia. By targeting CD20-positive B cells, these antibodies help to eliminate cancerous cells and control the progression of the disease.
- Common examples of anti-CD20 monoclonal antibodies include rituximab, obinutuzumab, and ofatumumab. These therapies have significantly advanced the treatment options for various immune-related conditions and cancers, offering targeted and often more tolerable alternatives to traditional chemotherapy.
- Market Dynamics:
Driving Factors:
- Anti-CD20 monoclonal antibodies have demonstrated high efficacy in the treatment of B-cell-related disorders, including autoimmune diseases and B-cell lymphomas. Their targeted approach to eliminating or modulating B cells has proven effective in managing these conditions.
- Continuous research and clinical trials have led to the discovery of new therapeutic indications for anti-CD20 monoclonal antibodies. As their effectiveness becomes established in treating a broader range of diseases, the market experiences growth driven by expanded applications.
- Ongoing advancements in biotechnology contribute to the development of novel and more efficient anti-CD20 monoclonal antibodies. Improved formulations, enhanced targeting mechanisms, and reduced side effects further drive the adoption of these therapies.
- The rising prevalence of B-cell lymphomas and other hematologic cancers contributes to the demand for anti-CD20 monoclonal antibodies. These antibodies play a crucial role in the treatment and management of various malignancies, driving market expansion.
- Regulatory approvals for new anti-CD20 monoclonal antibodies contribute significantly to market growth. The introduction of innovative therapies with improved efficacy and safety profiles attracts both healthcare professionals and patients.
Restrain Factors:
- Higher treatment costs.
- Limited oral formulations.
- Potential side-effects.
- Regulatory challenges.
- Risk of infections.
- Limited oral bio-availability.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/4678
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Report Scope:
| Attribute | Details |
| Market Size 2024 | US$ 9.0 billion |
| Projected Market Size 2034 | US$ 19.8 billion |
| CAGR Growth Rate | 9.0% |
| Market Segmentation | By Product Type- First Generation Anti-CD20 Monoclonal Antibodies, Second Generation Anti-CD20 Monoclonal Antibodies and Third Generation Anti-CD20 Monoclonal Antibodies By End-User- Hospitals, Clinics, Academic & Research Institutions and Contract Research Organization (CRO) |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Top Leading Players in Anti-CD20 Monoclonal Antibodies Market:
- Biogen, Inc.
- Genentech, Inc. (F. Hoffmann-La Roche AG)
- Genmab A/S
- Immunomedics, Inc.
- Novartis AG
- Spectrum Pharmaceuticals, Inc.
- Bio-Rad Laboratories, Inc.
- LFB Biotechnologies S.A.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/4678
Emerging Trends and Opportunities in Anti-CD20 Monoclonal Antibodies Market:
- Ongoing research and development efforts are focused on creating next-generation anti-CD20 monoclonal antibodies with enhanced efficacy, improved safety profiles, and novel mechanisms of action. The emergence of innovative therapies presents opportunities for differentiation and market expansion.
- Advancements in understanding the genetic and molecular characteristics of diseases allow for a more personalized approach to treatment. Tailoring anti-CD20 monoclonal antibody therapies based on individual patient profiles could optimize outcomes and minimize adverse effects.
- The exploration of combination therapies involving anti-CD20 monoclonal antibodies and other targeted agents or immunomodulatory drugs is gaining traction. Combinatorial approaches may improve treatment outcomes and address challenges such as resistance, offering new avenues for therapeutic intervention.
- The development and approval of biosimilar versions of established anti-CD20 monoclonal antibodies offer cost-effective alternatives. The biosimilars market presents opportunities for increased accessibility and competition, potentially driving down treatment costs.
- The collection and analysis of real-world evidence regarding the effectiveness and safety of anti-CD20 monoclonal antibodies in diverse patient populations can provide valuable insights. This evidence may guide treatment decisions, support regulatory submissions, and contribute to post-marketing surveillance.
- As healthcare infrastructure improves in emerging markets, there is an opportunity for the global expansion of anti-CD20 monoclonal antibodies. Increased awareness, regulatory harmonization, and partnerships can facilitate market entry into new regions.
Challenges of Anti-CD20 Monoclonal Antibodies Market:
- The cost of developing, manufacturing, and administering anti-CD20 monoclonal antibodies can be substantial. High treatment costs may limit accessibility for patients, strain healthcare budgets, and create disparities in access to effective therapies.
- Some patients may develop immune responses to monoclonal antibodies, leading to reduced efficacy or adverse reactions. The potential for immunogenicity poses challenges in ensuring consistent treatment outcomes and patient safety.
- Stringent regulatory requirements for the approval and commercialization of biologic therapies, including anti-CD20 monoclonal antibodies, can lead to delays in market entry. Navigating complex regulatory pathways poses challenges for manufacturers and may impact the timely availability of therapies.
- The immunosuppressive effects of anti-CD20 monoclonal antibodies may increase the risk of infections in treated individuals. Managing and mitigating the risk of infections requires close monitoring and poses challenges for healthcare providers.
- The manufacturing process for monoclonal antibodies is complex, involving specialized facilities and technologies. Ensuring consistent quality, meeting demand, and addressing manufacturing challenges pose ongoing hurdles for industry players.
Recent Development:
- In August 2018, Nippon Shinyaku Co. Ltd., and Chugai Pharmaceutical Co. Ltd., launched new obinutuzumab, the glycoengineered type II anti-CD20 monoclonal antibody brand name “GAZYVA Intravenous Infusion 1000mg” for treating CD20-positive follicular lymphoma. GAZYVA is confirmed to provide more additional benefits as compared to standard therapies in global Phase III GALLIUM study.
- Detailed Segmentation:
Anti-CD20 Monoclonal Antibodies Market, By Product Type:
- First Generation Anti-CD20 Monoclonal Antibodies
- Second Generation Anti-CD20 Monoclonal Antibodies
- Third Generation Anti-CD20 Monoclonal Antibodies
Anti-CD20 Monoclonal Antibodies Market, By End-User:
- Hospitals
- Clinics
- Academic & Research Institutions
- Contract Research Organizations (CRO’s)
Anti-CD20 Monoclonal Antibodies Market, By Region:
-
-
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
-
- Asia Pacific
-
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Mexico
- Rest of Latin America
-
- Middle East & Africa
-
- GCC
- Israel
- South Africa
- Rest of Middle East & Africa
-
- North America
-
- Key highlights of the Anti-CD20 Monoclonal Antibodies Market:
- The anti-CD20 monoclonal antibodies market has experienced rapid growth, driven by their effectiveness in treating various autoimmune diseases and B-cell-related cancers.
- Anti-CD20 monoclonal antibodies find applications in treating a broad spectrum of medical conditions, including rheumatoid arthritis, lupus, multiple sclerosis, and B-cell lymphomas.
- Ongoing research and clinical trials are exploring new therapeutic indications for anti-CD20 monoclonal antibodies, potentially broadening their usage beyond current approved indications.
- The development of next-generation anti-CD20 monoclonal antibodies is a significant trend, with a focus on improving efficacy, safety profiles, and patient outcomes.
- There is a growing emphasis on improving the overall patient experience during anti-CD20 monoclonal antibody therapy, from infusion processes to post-treatment support, to enhance treatment adherence and satisfaction.
- Research efforts are ongoing to better understand the mechanisms of action, improve treatment response predictability, and identify new therapeutic targets, driving continued innovation in the field.
- North America dominance - North America dominates the global market with the largest market share due to the presence of sophisticated healthcare infrastructure, proper reimbursement of therapy procedure, and increasing awareness about personalized medicines among the population.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/4678
Explore More Insights:
- Monoclonal Antibody Diagnostic Reagents Market - Trends, Analysis and Forecast till 2034
- Global Immuno-Oncology Drugs Market – Trends, Analysis and Forecast till 2034
- Molecular Diagnostics Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
