Dublin, April 19, 2024 (GLOBE NEWSWIRE) -- The "Clinical Trials Market, Size, Global Forecast 2024-2030, Industry Trends, Share, Growth, Insight, Impact of Inflation, Company Analysis" report has been added to ResearchAndMarkets.com's offering.
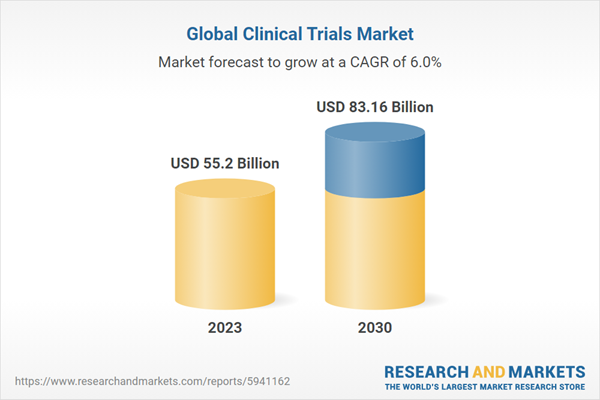
Global Clinical Trials Market is predicted to be valued at around US$ 83.16 Billion by 2030 from US$ 55.20 Billion in 2023, growing at a CAGR of 6.03% during 2024-2030
Currently, the clinical trials industry is evolving with technological advancements, streamlined regulations, and patient-centric approaches. AI, machine learning, and blockchain will transform clinical trials. They will make trials more efficient and cost-effective while increasing inclusivity and diversity. Precision medicine and biomarker-driven trials will enable tailored treatments for individual patients.
The growing burden of diseases needs more advanced and effective medicines for treatment, thus contributing to the market's growth. For instance, as per the IDF Atlas, there were 536.6 million people aged 20-79 living with diabetes globally, which is expected to reach 783.7 million by 2045. Such an excessive burden of diseases also drives the expansion of the market. Moreover, manufacturers' outsourcing of clinical research activities is one significant trend. By subcontracting their R&D projects, pharma and biotech businesses are reforming the drug development facilities business.
R&D service providers have grown from just a few establishments offering restricted clinical trial facilities to big conglomerates offering extensive facilities such as study design, preclinical assessments, clinical trial management and planning, autonomous safety data audits, and bio-statistical analysis. CROs (Contract Research Organizations) started by providing preclinical and clinical trial facilities. However, they are now proceeding with project administration.
The North American region is expected to contribute significantly to market growth during the study period owing to factors such as the high R&D expenditure of the pharmaceutical industry, the presence of well-established players, a robust regulatory framework, and the growing prevalence of diseases coupled with the significant contribution of the United States.
The strong foothold of the pharmaceutical industry, continuous advancements in clinical trial investigations, higher spending on research and development (R&D), growth of biosimilars and generics markets, and the rising trend of outsourcing preclinical, clinical, and laboratory testing services by pharmaceutical and biopharmaceutical companies in the region, have all contributed to this outcome. For instance, the National Cancer Institute (NCI) conducts clinical trials in the United States, regulated by the Food and Drug Administration (FDA). This federal enterprise gives funding for most U.S. cancer clinical tests.
Phase 3 trials hold the largest market share in global clinical trials
The global clinical trials market is split into phases: Phase 1, Phase 2, Phase 3, and Phase 4. The Phase III section is expected to lead the industry. Clinical trial statistics indicate market growth due to rising phase III trials involving many subjects. The average cost for a single-phase III trial is over USD 19.0 million. Also, phase III requires more patients and a more extended treatment period.
On the other hand, the Phase II section is anticipated to witness a considerable increase over the analysis period. Phase II plays an essential role, especially in oncology-related studies. The FDA estimated that around 33.0% of the investigational drugs are usually in phase II trials. Furthermore, numerous therapeutics and vaccines currently in phase II are indicated for oncology treatment, boosting market growth.
According to projections, oncology is expected to be the leading indication segment in the clinical trials market
By indication, the global clinical trials market is categorized into Autoimmune/Inflammation, Pain Management, Oncology, CNS Condition, Diabetes, Obesity, Cardiovascular, and Others. The oncology segment held the most extensive clinical trial market share. The large share can be attributed to the growing prevalence of cancer internationally and the high number of oncology clinical trials conducted globally.
For instance, as per the clinicaltrials.gov, for cancer remedy, there were about 106 early phase-I interventional trials in line, 2478 phase-I interventional trials were operative, 4219 phase-II interventional tests were active, 2031 phase-III interventional trials were in work, and 585 phase-IV interventional trials were active in 2022.
The interventional studies segment has been the undisputed global clinical trials market leader
By study material, the global clinical trials market is fragmented into Interventional, Observational, and Expanded Access. Interventional studies are projected to dominate the clinical trial market. The requirement for clinical trials to improve diagnostic tests and vaccines for viral illnesses such as SARS-CoV-2 has exponentially augmented the demand for clinical trials. Thus, the high incidence of novel viral diseases and ongoing technological improvements in clinical trials are significant reasons for the high revenue share of interventional studies.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 220 |
| Forecast Period | 2023 - 2030 |
| Estimated Market Value (USD) in 2023 | $55.2 Billion |
| Forecasted Market Value (USD) by 2030 | $83.16 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
Company Analysis:
- ICON Plc
- Wuxi AppTec
- SGS SA
- Syneos Health
- PRA Health Sciences Inc.
- Pfizer Inc.
- IQVIA
- Sanofi (France)
- Medpace
Phase - Global Clinical Trials Market breakup in 4 viewpoints:
- Phase 1
- Phase 2
- Phase 3
- Phase 4
Indication - Global Clinical Trials Market breakup in 8 viewpoints:
- Autoimmune/Inflammation
- Pain Management
- Oncology
- CNS Condition
- Diabetes
- Obesity
- Cardiovascular
- Others
Study Material - Global Clinical Trials Market breakup in 3 viewpoints:
- Interventional
- Observational
- Expanded Access
Countries - Global Clinical Trials Market breakup in 25 viewpoints:
- North America
- United States
- Canada
- Europe
- France
- Germany
- Italy
- Spain
- United Kingdom
- Belgium
- Netherlands
- Turkey
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Malaysia
- Indonesia
- Australia
- New Zealand
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- Saudi Arabia
- United Arab Emirates
- South Africa
For more information about this report visit https://www.researchandmarkets.com/r/ckxhpt
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
