New York, USA, Aug. 12, 2024 (GLOBE NEWSWIRE) -- PROTAC Market is Expected to Showcase Significant Growth During the Study Period (2020–2034) | DelveInsight
The PROTAC market is anticipated to expand considerably in the near future, driven by the rising number of PROTAC-based drugs undergoing clinical trials from multiple companies. At present, no PROTAC technology-based therapies have been approved.
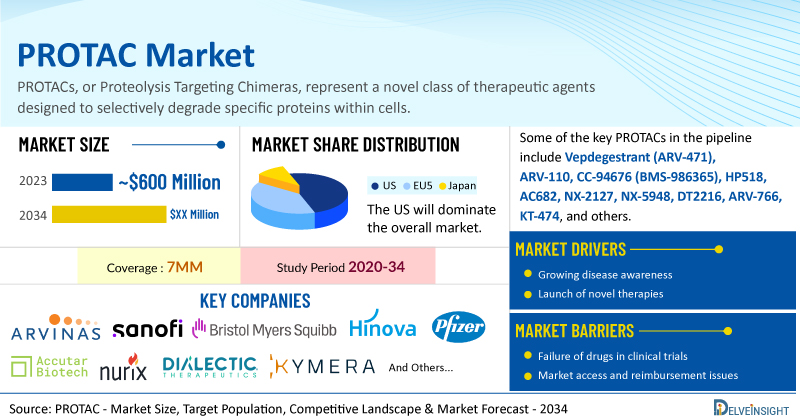
DelveInsight’s PROTAC Market Insights report includes a comprehensive understanding of current treatment practices, emerging PROTACs, market share of individual therapies, and current and forecasted PROTAC market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the PROTAC Market Report
- As per DelveInsight’s analysis, the PROTAC market is anticipated to grow at a significant CAGR by 2034.
- Leading PROTAC companies such as Arvinas, Pfizer, BMS, Hinova Pharmaceuticals, Accutar Biotech, Nurix Therapeutics, Dialectic Therapeutics, Kymera, Sanofi, and others are developing novel PROTACs that can be available in the PROTAC market in the coming years.
- Some of the key PROTACs in the pipeline include Vepdegestrant (ARV-471), ARV-110, CC-94676 (BMS-986365), HP518, AC682, NX-2127, NX-5948, DT2216, ARV-766, KT-474, and others.
- Many Pharma companies presented the data of their PROTAC during the ASCO GU 2024 conference including Hinova Pharmaceuticals and others. Hinova study’s concluded that HP518 is a novel AR PROTAC degrader and shows favorable safety/tolerability and efficacy signals in unselected mCRPC patients. AR LBD mutations may indicate potential benefit from HP518, warranting further investigation.
- In February 2024, vepdegestrant received FDA Fast Track Designation for the treatment of adult patients with ER+/HER2- locally advanced or metastatic breast cancer that received prior treatment with endocrine-based therapy as a monotherapy.
- In February 2024, Arvinas announced the first-in-human dosing of ARV-102, an investigational PROTAC protein degrader for neurodegenerative disease. It is designed to cross the blood-brain barrier and target leucine-rich repeat kinase 2 (LRRK2).
Discover which therapies are expected to grab the PROTAC market share @ PROTAC Market Report
The market for PROTACs is growing rapidly, driven by advancements in drug discovery technologies and increasing investment in research and development. One of the primary factors influencing the PROTAC market dynamics is the substantial investment from pharmaceutical and biotechnology companies. Major players in the industry are pouring resources into PROTAC research to capitalize on its potential advantages over traditional small molecules and biologics.
This influx of capital is fostering innovation and accelerating the development of new PROTAC-based therapies. Additionally, strategic collaborations between academic institutions and industry leaders are further propelling the market forward, as these partnerships often lead to breakthroughs in PROTAC technology and its applications.
The competitive landscape of the PROTAC market is characterized by both established pharmaceutical companies and emerging biotech startups. This diversity of players contributes to a vibrant and rapidly evolving market environment. Companies are focusing on developing proprietary PROTAC platforms, optimizing delivery mechanisms, and expanding the range of target proteins that can be effectively degraded. Moreover, the entry of multiple stakeholders fosters a competitive atmosphere that drives innovation and speeds up the development process, ultimately benefiting patients through more effective and targeted treatments.
Despite the promising prospects, the PROTAC market also faces several challenges. One significant hurdle is the complexity of PROTAC development and manufacturing processes, which can be costly and time-consuming. Additionally, there are scientific and regulatory challenges associated with ensuring the safety and efficacy of PROTAC-based therapies. Navigating these challenges requires a concerted effort from researchers, regulatory agencies, and industry leaders to address potential issues and facilitate the successful translation of PROTACs from the lab to the clinic.
In summary, the PROTAC market is positioned for substantial growth due to its innovative approach to targeted protein degradation and the increasing investment from various stakeholders. While there are challenges to overcome, the dynamic nature of the market and ongoing advancements in technology and research are likely to drive continued progress and expansion in this promising field.
PROTAC Treatment Market
PROTAC doesn't directly eliminate proteins but instead leverages the ubiquitin-proteasome system (UPS) – the cell's natural mechanism for protein degradation. PROTAC molecules have two components connected by a linker: one part, the target binder, attaches to a specific protein similar to traditional small molecules, but this binding doesn’t necessarily inhibit the protein's function. The other part of PROTAC interacts with an enzyme called E3 ubiquitin ligase, bringing it into proximity with the target protein. This interaction leads the E3 ligase to tag the protein for degradation by the proteasome, the cellular "waste disposal machine" that breaks down unnecessary or damaged proteins. Due to their unique mechanism, PROTACs offer several advantages over conventional small-molecule drugs.
Learn more about the FDA-approved PROTAC @ PROTAC Drugs
Key Emerging PROTACs and Companies
Several key players, including Pfizer/Arvinas, Celgene/BMS, Hinova, and others, are involved in developing drugs for PROTAC for various indications such as ER+/HER2-Breast Cancer, Prostate Cancer Metastatic, Metastatic Castration-resistant Prostate Cancer and others.
Vepdegestrant is an experimental oral PROTAC protein degrader, developed jointly by Arvinas and Pfizer. Arvinas employs its proprietary PROTAC Discovery Engine platform to create proteolysis-targeting chimeras, or PROTAC-targeted protein degraders, which aim to utilize the body's natural protein disposal mechanisms to selectively and effectively eliminate disease-causing proteins.
Currently, Vepdegestrant is being assessed as a standalone treatment in the second-line setting through the ongoing Phase III VERITAC-2 clinical trial, and as a first-line treatment combined with palbociclib in the lead-in cohort of the Phase III VERITAC-3 clinical trial. It is also being tested for potential use in combination therapies with abemaciclib, ribociclib, samuraciclib, everolimus, and Pfizer’s experimental novel CDK4 inhibitor, PF-07220060.
ARV-110 is an orally available PROTAC designed to target and degrade the androgen receptor (AR) protein. It is a bifunctional molecule that enables the interaction between the androgen receptor and an intracellular E3 ligase complex, leading to the receptor's ubiquitination and subsequent degradation via the proteasome. Developed by Arvinas, ARV-110 is being investigated as a potential treatment for men with metastatic castration-resistant prostate cancer. The drug aids in the ubiquitination and degradation of androgen receptors and is used in prostate cancer research. In March 2024, Arvinas announced plans to discuss the alignment of the Phase III trial for hormone-refractory, metastatic prostate cancer (second-line therapy or beyond) with regulatory authorities in the second quarter of 2024.
The other PROTAC-based therapies in the pipeline include
- CC-94676 (BMS-986365): Celgene/BMS
- HP518: Hinova Pharmaceuticals
- AC682: Accutar Biotech
- NX-2127: Nurix Therapeutics
- NX-5948: Nurix Therapeutics
- DT2216: Dialectic Therapeutics
- ARV-766: Arvinas
- KT-474: Kymera/Sanofi
The anticipated launch of these emerging therapies are poised to transform the PROTACs market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the PROTACs market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about PROTAC clinical trials, visit @ PROTAC Treatment Drugs
PROTAC Overview
PROTACs, or Proteolysis Targeting Chimeras, represent a novel class of therapeutic agents designed to selectively degrade specific proteins within cells. Unlike traditional small molecules that inhibit protein function, PROTACs harness the cell’s own ubiquitin-proteasome system to target and degrade proteins. This approach involves a bifunctional molecule with two distinct binding domains: one that recognizes the target protein and another that binds to an E3 ligase, an enzyme responsible for tagging proteins for degradation. By bridging these two components, PROTACs facilitate the ubiquitination and subsequent destruction of the target protein, thereby offering a powerful strategy for modulating protein levels and overcoming challenges associated with conventional therapies.
The potential applications of PROTACs in drug discovery and development are vast. They provide a means to address previously "undruggable" targets by effectively reducing their levels rather than merely inhibiting their activity. This approach is particularly promising for diseases driven by aberrant or overexpressed proteins, such as certain cancers and neurodegenerative disorders. As research advances, PROTAC technology could revolutionize how we tackle complex diseases by offering more precise and effective therapeutic options, potentially leading to improved outcomes and fewer side effects compared to traditional drugs.
| PROTAC Report Metrics | Details |
| Study Period | 2020–2034 |
| PROTAC Report Coverage | 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key PROTAC Companies | Arvinas, Pfizer, BMS, Hinova Pharmaceuticals, Accutar Biotech, Nurix Therapeutics, Dialectic Therapeutics, Kymera, Sanofi, and others |
| Key PROTAC | Vepdegestrant (ARV-471), ARV-110, CC-94676 (BMS-986365), HP518, AC682, NX-2127, NX-5948, DT2216, ARV-766, KT-474, and others |
Scope of the PROTAC Market Report
- PROTAC Therapeutic Assessment: PROTAC current marketed and emerging therapies
- PROTAC Market Dynamics: Conjoint Analysis of Emerging PROTAC Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, PROTAC Market Access and Reimbursement
Discover more about PROTAC drugs in development @ PROTAC Clinical Trials
Table of Contents
| 1. | PROTAC Market Key Insights |
| 2. | PROTAC Market Report Introduction |
| 3. | Executive Summary of PROTACs |
| 4. | Key Events |
| 5. | PROTAC Market Forecast Methodology |
| 6. | PROTACs Market Overview at a Glance in the 7MM |
| 7. | PROTACs: Background and Overview |
| 8. | PROTACs Target Patient Pool |
| 9. | PROTAC Marketed Drugs |
| 10. | PROTAC Emerging Drugs |
| 11. | Seven Major PROTAC Market Analysis |
| 12. | PROTAC Market Access and Reimbursement |
| 13. | SWOT Analysis |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | Appendix |
| 17. | DelveInsight Capabilities |
| 18. | Disclaimer |
| 19. | About DelveInsight |
Related Reports
Metastatic HER2-Positive Breast Cancer Market
Metastatic HER2-Positive Breast Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key metastatic HER2-positive breast cancer companies including Byondis, Roche, Ambrx, Zymeworks, Jazz Pharmaceuticals, Pfizer, among others.
Metastatic HR+/HER2− Breast Cancer Market
Metastatic HR+/HER2− Breast Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key metastatic HR+/HER2-negative breast cancer companies including Merck, Arvinas, Olema Pharmaceuticals, Celcuity, Roche, AstraZeneca, Daiichi Sankyo, Eli Lilly, Sermonix Pharmaceuticals, Genentech, Veru Pharma, DualityBio, BioNtech, Evgen Pharma, Carrick Therapeutics, EQRx, G1 Therapeutics, Immutep, among others.
Metastatic Prostate Cancer Market
Metastatic Prostate Cancer Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key metastatic prostate cancer companies, including AstraZeneca, Merck Sharp & Dohme, Hinova Pharmaceuticals, Pfizer, Astellas Pharma, Modra Pharmaceuticals, AB Science, Eli Lilly and Company, Zr Pharma & GmbH, Bristol-Myers Squibb, Ipsen, Exelixis, Takeda, Janssen Research & Development, Tesaro, Lantheus Holdings, Kintor Pharmaceutical, MacroGenics, Daiichi Sankyo, Madison Vaccines, Novartis, Point Biopharma, Xencor, Essa Pharma, Telix International, Bayer, Arvinas, among others.
Metastatic Castration-Resistant Prostate Cancer Market
Metastatic Castration-Resistant Prostate Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key metastatic castration-resistant prostate cancer companies including AstraZeneca, Arvinas, Madison Vaccines, Phosplatin Therapeutics, Hinova Pharmaceuticals, Bristol Myers Squibb, Merck, MacroGenics, Daiichi Sankyo, Seagen, Taiho Pharmaceutical, Modra Pharmaceuticals, Xencor, Point Biopharma, Lantheus Holdings, Zenith Epigenetics, Essa Pharma, Telix Pharmaceuticals, Kintor Pharmaceutical, AB Science, Eli Lilly and Company, Exelixis among others.
Metastatic Hormone-Refractory Prostate Cancer Pipeline
Metastatic Hormone-Refractory Prostate Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key metastatic hormone-refractory prostate cancer companies, including AstraZeneca, Arvinas, Madison Vaccines, Phosplatin Therapeutics, Hinova Pharmaceuticals, Bristol Myers Squibb, Merck, MacroGenics, Daiichi Sankyo, Seagen, Taiho Pharmaceutical, Modra Pharmaceuticals, Xencor, Point Biopharma, Lantheus Holdings, Zenith Epigenetics, Essa Pharma, Telix Pharmaceuticals, Kintor Pharmaceutical, AB Science, Eli Lilly and Company, Exelixis, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
