Great Basin Scientific Receives FDA 510(k) Clearance for Stool Bacterial Pathogens Panel
July 13, 2017 07:00 ET
|
Great Basin Scientific, Inc.
SALT LAKE CITY, July 13, 2017 (GLOBE NEWSWIRE) -- Great Basin Scientific, Inc. (OTCQB:GBSN), a molecular diagnostics company, today announced it has received U. S. Food and Drug Administration (FDA)...
Intuitive Surgical Receives FDA Clearance for Latest da Vinci® Robotic-Assisted Surgical System
May 30, 2017 16:31 ET
|
Intuitive Surgical, Inc.
SUNNYVALE, Calif., May 30, 2017 (GLOBE NEWSWIRE) -- Intuitive Surgical, a global technology leader in robotic-assisted, minimally invasive surgery, announced today that its new da Vinci X Surgical...
Great Basin Scientific Receives FDA 510(k) Clearance for Bordetella Direct Test
April 03, 2017 16:00 ET
|
Great Basin Scientific, Inc.
SALT LAKE CITY, April 03, 2017 (GLOBE NEWSWIRE) -- Great Basin Scientific, Inc. (OTCQB:GBSN), a molecular diagnostics company, has received U.S. Food & Drug Administration (FDA) clearance for...
biotricity Receives an FDA 510(k) Clearance – Marks Major Milestone in Path to Commercialization
October 18, 2016 08:00 ET
|
biotricity
REDWOOD CITY, Calif., Oct. 18, 2016 (GLOBE NEWSWIRE) -- biotricity inc. (OTCQB:BTCY), a healthcare technology company dedicated to delivering innovative, medically relevant biometric remote...
Ekso Bionics® To Showcase First FDA Cleared Exoskeleton Approved for Use with Stroke Patients at 9th World Congress for NeuroRehabilitation (WCNR)
May 10, 2016 07:00 ET
|
Ekso Bionics Holdings, Inc.
RICHMOND, Calif., May 10, 2016 (GLOBE NEWSWIRE) -- Ekso Bionics Holdings, Inc. (OTCQB:EKSOD), a robotic exoskeleton company, today announced that it will be attending the 9th World Congress for...
FDA Clears Parker’s Indego® Exoskeleton for Clinical, Personal Use
March 10, 2016 09:15 ET
|
Parker-Hannifin Corporation
CLEVELAND, March 10, 2016 (GLOBE NEWSWIRE) -- Parker Hannifin Corporation (NYSE: PH), the global leader in motion and control technologies, today announced that the U.S. Food and Drug...
MEDTECH Receives FDA Clearance for ROSA Spine
January 05, 2016 17:00 ET
|
Medtech SA
MONTPELLIER, France, Jan. 05, 2016 (GLOBE NEWSWIRE) -- MEDTECH (Euronext, FR0010892950 – ROSA), a company specialized in designing, developing and marketing innovative surgical assistance robots,...
GI Logic Receives FDA 510K Clearance for the AbStats™ System for Real-Time Digestive Telemetry
December 18, 2015 10:19 ET
|
GI Logic
PASADENA, Calif., Dec. 18, 2015 (GLOBE NEWSWIRE) -- GI Logic, a privately held gastroenterology medical device company, announced today it has received FDA 510K clearance for the AbStats™ System...
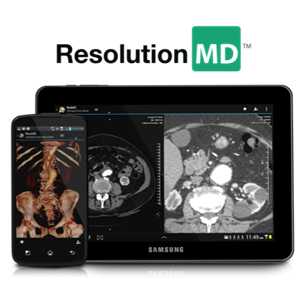
Calgary Scientific's ResolutionMD(TM) First to Receive FDA Clearance for Diagnostic Medical Image Viewing on Android Devices
April 09, 2013 09:01 ET
|
Calgary Scientific, Inc.
CALGARY, Alberta, Canada, April 9, 2013 (GLOBE NEWSWIRE) -- Calgary Scientific Inc., a company known for creating transformative technology for the medical industry and beyond, has taken a huge step...
CARESIDE On Schedule with FDA Point-of-Care Marketing Submission
November 02, 1999 15:21 ET
|
Careside, Inc
CULVER CITY, Calif. November 2, 1999 (PRIMEZONE) -- CARESIDE, Inc. (AMEX:CSA) (AMEX:CSA.W) announced its filing of a premarket notification to the U.S. Food and Drug Administration (FDA) to add the...