New York, USA, July 31, 2024 (GLOBE NEWSWIRE) -- Fuchs Endothelial Corneal Dystrophy Market to Exhibit Promising Growth During the Study Period (2020–2034) | DelveInsight
The market for Fuchs endothelial corneal dystrophy is expected to boost in the coming years. This growth can be attributed to the introduction of upcoming therapies and the rising prevalence of the disease. The anticipated launch of these therapies is also expected to attract new entrants to the FECD market, resulting in increased competition and innovation.
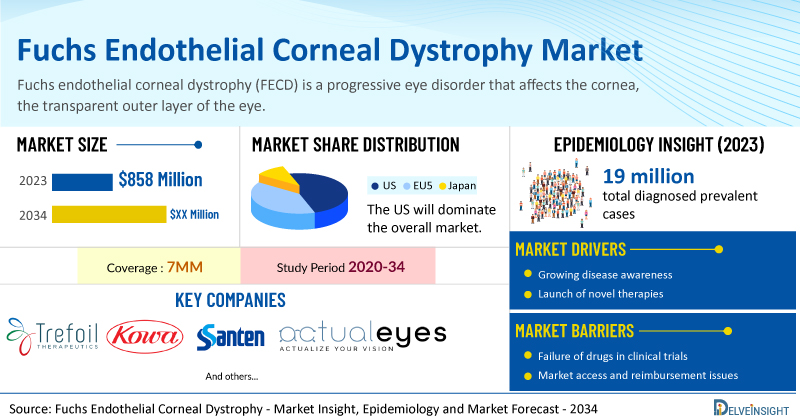
DelveInsight’s Fuchs Endothelial Corneal Dystrophy Market Insights report includes a comprehensive understanding of current treatment practices, Fuchs endothelial corneal dystrophy emerging drugs, market share of individual therapies, and current and forecasted Fuchs endothelial corneal dystrophy market size from 2020 to 2034, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Fuchs Endothelial Corneal Dystrophy Market Report
- According to DelveInsight’s analysis, the market size of FECD in the 7MM reached USD 858 million in 2023 and it is expected to grow at a significant CAGR by 2034.
- As per DelveInsight analysis, in 2023, the total diagnosed prevalent cases of FECD in the 7MM were ~19 million. These cases are projected to increase further by 2034.
- Prominent companies working in the domain of FECD, including Kowa Pharmaceuticals, Trefoil Therapeutics, Santen, ActualEyes, and others, are actively working on innovative drugs for FECD. These novel FECD therapies are anticipated to enter the FECD market in the forecast period and are expected to change the market.
- Some of the key therapies for FECD treatment include Ripasudil (K-321), TTHX1114, STN1010904/AE-001 (sirolimus), and others.
Discover which therapies are expected to grab FECD market share @ Fuchs Endothelial Corneal Dystrophy Market Report
Fuchs Endothelial Corneal Dystrophy Overview
Fuchs endothelial corneal dystrophy (FECD) is a progressive eye disorder that affects the cornea, the transparent outer layer of the eye. It's characterized by the gradual deterioration of endothelial cells, which are responsible for maintaining the cornea's hydration and clarity. While the exact cause of FECD remains unclear, it is believed to have both genetic and environmental factors contributing to its development.
Individuals with FECD often experience symptoms such as blurry or cloudy vision, particularly in the morning or after periods of extended rest due to corneal swelling overnight. Other common symptoms include glare, halos around lights, and decreased visual acuity, which can significantly impact daily activities and quality of life.
Diagnosing FECD typically involves a comprehensive eye examination, including a visual acuity test, slit-lamp examination to assess the corneal thickness and clarity, and measurement of corneal endothelial cell density. Additionally, specular microscopy may be utilized to examine the structure of endothelial cells and evaluate the severity of the condition.
Fuchs Endothelial Corneal Dystrophy Epidemiology Segmentation
The FECD epidemiology section provides insights into the historical and current FECD patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The FECD market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Total Diagnosed Prevalent Cases of Fuchs Endothelial Corneal Dystrophy
- Gender-specific Cases of Fuchs Endothelial Corneal Dystrophy
- Age-specific Cases of Fuchs Endothelial Corneal Dystrophy
- Grade-specific Cases of Fuchs Endothelial Corneal Dystrophy
Download the report to understand which factors are driving FECD epidemiology trends @ Fuchs Endothelial Corneal Dystrophy Epidemiological Insights
Fuchs Endothelial Corneal Dystrophy Treatment Market
The medical approach to early FECD involves reducing corneal swelling through the use of various topical treatments like sodium chloride 5% drops, hypertonic saline drops, and solutions such as octasiloxane or ointments. These aim to lessen morning edema and aid in corneal dehydration. Additionally, procedures like phototherapeutic keratectomy, amniotic membrane transplants, and anterior stromal puncture are employed to alleviate painful symptoms, particularly those linked with ruptured bullae in the later stages of the disease. Even methods like cycloplegic administration, antibiotic ointment application, and patching are utilized to manage ruptured corneal bullae. For persistent or sizable epithelial defects, bandage contact lenses are often recommended.
However, it's important to note that these treatments don't address the root cause of the disease, which is endothelial layer dysfunction. They only offer temporary relief and don't alter the disease's progression. Furthermore, these agents can cause stinging, which may affect their acceptance by patients.
Corneal transplantation using healthy donor endothelial tissue is the primary treatment for Fuchs dystrophy. This involves replacing the damaged endothelium and Descemet’s membrane with healthy cells from a donor. Over the past twenty years, there have been significant advancements in surgical techniques, instruments, medications, and eye bank practices for managing endothelial dysfunction. These improvements have led to a decrease in the need for complete corneal transplantation, with a rise in procedures like lamellar keratoplasty that target specific areas of corneal dysfunction.
Surgical techniques have evolved from traditional penetrating keratoplasty, which involved replacing the entire cornea, to more refined procedures such as endothelial keratoplasty, Descemet's stripping automated endothelial keratoplasty (DASEK), and Descemet membrane endothelial keratoplasty (DMEK). In the past, patients with Fuchs dystrophy often had to wait until their condition became severe before undergoing PK. However, with the introduction of procedures like posterior lamellar keratoplasty (PLK) or deep lamellar endothelial keratoplasty (DLEK), which involve replacing only the posterior layers of the cornea, including the Descemet's membrane and endothelium, through a smaller incision, surgical intervention can now be offered earlier in the disease process.
Learn more about the FDA-approved drugs for FECD @ Drugs for Fuchs Endothelial Corneal Dystrophy Treatment
Fuchs Endothelial Corneal Dystrophy Emerging Drugs and Companies
The expected launch of emerging therapies, such as Ripasudil (K-321) (Kowa Pharmaceuticals), TTHX1114 (Trefoil Therapeutics), and STN1010904/AE-001 (sirolimus) (Santen/ActualEyes), and others are expected to create a positive impact on the market. Other therapies in the early stages of the trial are also being developed.
Ripasudil, a rho-kinase inhibitor, reduces intraocular pressure (IOP) by enhancing the conventional outflow of aqueous humor. Rho-associated protein kinase (ROCK) plays a crucial role in cell shape and movement regulation across various tissues, including the eye. By targeting ROCK, this inhibitor disrupts the intracellular actin structure in the Trabecular Meshwork (TM) and Schlemm’s canal (SC), key components of the conventional outflow pathway, thereby lowering IOP. Additionally, it inhibits the production of extracellular matrix by TM cells, offering a promising alternative for IOP reduction. Currently, Ripasudil is undergoing Phase III clinical trials for treating FECD.
TTHX1114, a specialized form of fibroblast growth factor-1 (eFGF-1), is engineered to safeguard corneal endothelial cells from harm and stress, thereby aiding in the restoration of vision loss by prompting cell growth and movement. The development of TTHX1114 has been significantly supported by the NIH through its Therapeutics for Rare and Neglected Diseases Program. Trefoil Therapeutics recently revealed encouraging outcomes from the Phase II (STORM) trial of TTHX1114, demonstrating its efficacy in regenerating the cornea and restoring vision post-DSO surgery. Additionally, Trefoil is working on a topical version of TTHX1114, which aims to foster the growth of corneal epithelial cells, potentially mitigating complications like pain, inflammation, and vision loss associated with ulcerative corneal conditions.
STN1010904, also referred to as sirolimus or AE-001, is a medication in ophthalmic suspension form created by Santen Pharmaceutical in collaboration with ActualEyes. It targets FECD, acting as a sirolimus eye drop that inhibits mTOR, thereby reducing the formation of collagenous deposits on the corneal endothelial layer and preventing endothelial cell apoptosis associated with FECD. By alleviating corneal edema, it preserves corneal clarity and visual sharpness. Santen Pharmaceuticals and ActualEyes are jointly developing this treatment to ensure its timely availability, aiming to address the needs of FECD patients and enhance their quality of life. Phase II trials began in the US, France, and India in May 2022, with plans for completion by 2025.
The anticipated launch of these emerging therapies are poised to transform the FECD market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the FECD market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about FECD clinical trials, visit @ Fuchs Endothelial Corneal Dystrophy Treatment Drugs
Fuchs Endothelial Corneal Dystrophy Market Dynamics
The Fuchs endothelial corneal dystrophy market dynamics are anticipated to change in the coming years. Advancements in understanding the pathophysiology and genetics of FECD have led to the identification of new treatment targets, contributing to the development of personalized medicine for patients, which is crucial for better management of the indication, particularly for early-stage patients not yet eligible for surgery; furthermore, safe and effective gene therapy is needed, given that genetic factors are the major cause of FECD, and therapies utilizing CRISPR/Cas approaches, currently in initial stages of development, could provide an ideal solution, potentially eliminating the need for transplantation.
Furthermore, many potential therapies are being investigated for the treatment of FECD, and it is safe to predict that the treatment space will significantly impact the FECD market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of the FECD market in the 7MM.
However, several factors may impede the growth of the FECD market. The lack of universal guidelines regarding disease diagnosis and treatment impacts the treatment regime for the patient, making it difficult to diagnose FECD at an early stage due to its late onset nature and variable clinical presentation, which in turn limits the availability of accurate prevalence figures for the disease, with a significant limitation being the supply of donor corneas; additionally, traditional corneal specialists may oppose the adoption of new therapies like cell therapies due to their less established safety and efficacy.
Moreover, FECD treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, FECD market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact FECD market growth.
| Fuchs Endothelial Corneal Dystrophy Report Metrics | Details |
| Study Period | 2020–2034 |
| Fuchs Endothelial Corneal Dystrophy Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Fuchs Endothelial Corneal Dystrophy Market Size in 2023 | USD 858 Million |
| Key Fuchs Endothelial Corneal Dystrophy Companies | Kowa Pharmaceuticals, Trefoil Therapeutics, Santen, ActualEyes, and others |
| Key Fuchs Endothelial Corneal Dystrophy Therapies | Ripasudil (K-321), TTHX1114, STN1010904/AE-001 (sirolimus), and others |
Scope of the Fuchs Endothelial Corneal Dystrophy Market Report
- Fuchs Endothelial Corneal Dystrophy Therapeutic Assessment: Fuchs Endothelial Corneal Dystrophy current marketed and emerging therapies
- Fuchs Endothelial Corneal Dystrophy Market Dynamics: Conjoint Analysis of Emerging Fuchs Endothelial Corneal Dystrophy Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Fuchs Endothelial Corneal Dystrophy Market Access and Reimbursement
Discover more about FECD drugs in development @ Fuchs Endothelial Corneal Dystrophy Clinical Trials
Table of Contents
| 1. | Fuchs Endothelial Corneal Dystrophy Market Key Insights |
| 2. | Fuchs Endothelial Corneal Dystrophy Market Report Introduction |
| 3. | Fuchs Endothelial Corneal Dystrophy Market Overview at a Glance |
| 4. | Fuchs Endothelial Corneal Dystrophy Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Fuchs Endothelial Corneal Dystrophy Treatment and Management |
| 7. | Fuchs Endothelial Corneal Dystrophy Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Fuchs Endothelial Corneal Dystrophy Marketed Drugs |
| 10. | Fuchs Endothelial Corneal Dystrophy Emerging Drugs |
| 11. | Seven Major Fuchs Endothelial Corneal Dystrophy Market Analysis |
| 12. | Fuchs Endothelial Corneal Dystrophy Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Fuchs Endothelial Corneal Dystrophy Epidemiology Forecast
Fuchs Endothelial Corneal Dystrophy Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted FECD epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Fuchs Endothelial Corneal Dystrophy Pipeline
Fuchs Endothelial Corneal Dystrophy Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key FECD companies, including Trefoil Therapeutics, Kowa Pharmaceutical, Santen Pharmaceutical, among others.
Bullous Keratopathy Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key bullous keratopathy companies, including Trefoil Therapeutics, Emmecell, Cellusion, among others.
Presbyopia Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key presbyopia companies, including Eyenovia, Orasis Pharmaceuticals, Ocuphire Pharma, Visus Therapeutics, LENZ Therapeutics, Glaukos Corporation, AbbVie, among others.
Presbyopia Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key presbyopia companies, including Orasis Pharmaceuticals, Novartis, Cellix Bio, Visus Therapeutics, AbbVie, Vyluma, Lenz Therapeutics, Ocuphire Pharma, JIXING Pharmaceuticals, Eyenovia, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
