Arch Therapeutics Receives Notice of Allowance for Composition of Matter and Method of Use Patent Covering Solid Forms of Self-Assembling Peptides
10 mai 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - May 10, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
Arch Therapeutics to Present at PIONEERS 2016 by Joseph Gunnar & Co. Thursday, May 5, 2016
26 avr. 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Apr 26, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
Arch Therapeutics Receives Notice of Allowance for Patent Covering Systemic Applications for Self-Assembling Peptides
25 avr. 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Apr 25, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
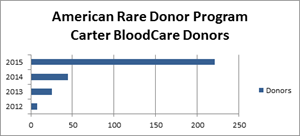
Carter BloodCare one of only two U.S. laboratories to receive AABB accreditation for molecular testing for red cell antigens
21 avr. 2016 18h14 HE
|
Carter BloodCare
DALLAS and FORT WORTH, Texas, April 21, 2016 (GLOBE NEWSWIRE) -- Carter BloodCare, a blood center serving 200 medical facilities in 50-plus Texas counties, announces it is only the second blood center...
Arch Therapeutics Announces Notice of Allowance for Method of Use Patent Covering Uses of Self-Assembling Peptides
11 avr. 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Apr 11, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
Arch Therapeutics Announces Notice of Allowance for Composition-of-Matter Patent Covering Self-Assembling Peptides
05 avr. 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Apr 5, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
Arch Therapeutics Obtains Favorable Safety Data for AC5 Surgical Hemostatic Device(TM) in Skin Irritation Testing in Humans
22 mars 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Mar 22, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ ("AC5™"), reports...
Arch Therapeutics Reports Favorable Results for AC5 Surgical Hemostatic Device in Biocompatibility Testing Required for CE Mark
14 mars 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Mar 14, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ ("AC5™"), has obtained...
Arch Therapeutics to Present at the 28th Annual Roth Capital Conference on Tuesday, March 15, 2016
10 mars 2016 08h20 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Mar 10, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ (AC5™) for use in...
Arch Therapeutics Receives ISO 13485 Certification for AC5
09 mars 2016 07h57 HE
|
Arch Therapeutics, Inc.
FRAMINGHAM, MA--(Marketwired - Mar 9, 2016) - Arch Therapeutics, Inc. (OTCQB: ARTH) ("Arch" or the "Company"), developer of the AC5 Surgical Hemostatic Device™ ("AC5™"), today announces...