SALT LAKE CITY, Feb. 10, 2016 (GLOBE NEWSWIRE) -- ARUP Laboratories, a leading reference laboratory and a nonprofit enterprise of the University of Utah and its Department of Pathology, updated their Cytochrome P450 Genotype Panel test in an effort to prevent adverse drug reactions, decrease emergency room and hospital visits, and advance personalized medicine.
ARUP will now include Coriell Life Sciences' (CLS) GeneDose—a comprehensive medication risk and response report.
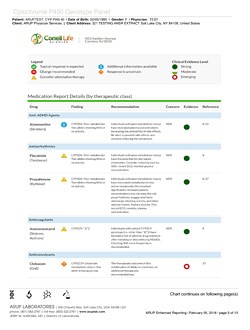
The ARUP clinical genetic test is specialized to DNA that directly impacts drug safety and efficacy. The test results are communicated by GeneDose—a convenient report and decision support tool for rapid and efficient physician use at the point of care.
The complementary interactive (real-time) risk-management tool, GeneDose LIVE, builds on the static medication response report to provide physicians, pharmacists, and healthcare providers with a rapid medication appropriateness assessment based on their patient's personal information, therapeutic regimen, medical conditions, and dietary supplements. The tool can explore alternative medications in search of the best risk profile. It supports the treatment of polypharmacy patients at risk of adverse drug events, individuals with a history of medication therapy failures, elderly and historically complicated patients, and children who have no personal history with medications.
"We're increasingly focused on providing our clients with a deep, information-rich context for the test results they receive," says Dr. Brian Jackson, VP, Chief Medical Informatics Officer. "This knowledge allows clinicians quick access to comprehensive information, including references to evidence-based research, to guide them in patient-care."
ARUP's CYP Panel test includes the genes that predict drug-metabolizing enzyme activity for many commonly administered drugs. "This test will help identify those people who are at increased risk for therapeutic failure and/or toxicity from certain drugs," says Gwen McMillin, PhD, medical director of the Toxicology Laboratory and co-medical director of Pharmacogenetics at ARUP. "We recognized that CLS is the right partner to communicate complex genetic information to our clients."
GeneDose tools leverages input from independent experts in medical genetics, information gleaned from eight years of research in the Coriell Personalized Medicine Collaborative study, the curation of hundreds of research studies, data from pharmaceutical companies, the US FDA, the Clinical Pharmacogenetics Implementation Consortium, and other trusted scientific sources.
"We are thrilled to be a part of ARUP's continued growth in pharmacogenomics and personalized medicine," says Scott Megill, President and CEO of CLS. "ARUP is a national leader in laboratory genetics and together we will improve health outcomes at the point-of-care."
"Doctors are pressed for time with each patient as it is—these clinical tools will improve the patient-doctor interaction while alerting doctors to medication misadventures."
About ARUP Laboratories
Founded in 1984, ARUP Laboratories is a leading national reference laboratory and a nonprofit enterprise of the University of Utah and its Department of Pathology. ARUP offers more than 3,000 tests and test combinations, ranging from routine screening tests to esoteric molecular and genetic assays. ARUP serves clients across the United States, including many of the nation's top university teaching hospitals and children's hospitals, as well as multihospital groups, major commercial laboratories, group purchasing organizations, military and other government facilities, and major clinics. In addition, ARUP is a worldwide leader in innovative laboratory research and development, led by the efforts of the ARUP Institute for Clinical and Experimental Pathology®.
About Coriell Life Sciences
Coriell Life Sciences (CLS) is a cutting-edge, for-profit venture focused on empowering physicians to apply genetic analyses at point-of-care. CLS is a spin-off of the internationally-renowned Coriell Institute for Medical Research, a non-profit biomedical research institution dedicated to studying the human genome to improve the lives of healthcare consumers. The organization leverages the framework of the Coriell Personalized Medicine Collaborative, an innovative research study that brings together more than 7,000 study participants, physicians, scientists, ethicists, genetic counselors, and information technologists.
Photos accompanying this release are available at:
http://www.globenewswire.com/newsroom/prs/?pkgid=38900
http://www.globenewswire.com/newsroom/prs/?pkgid=38901