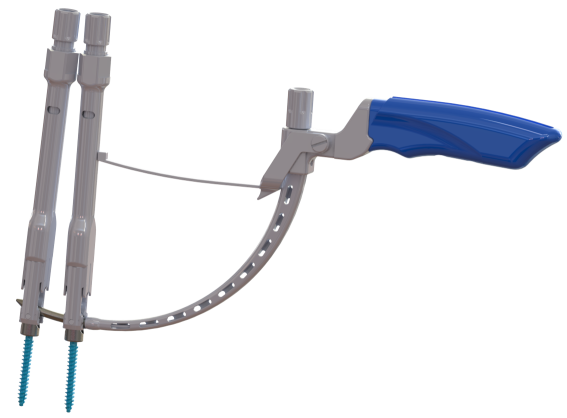
FLORENCE, S.C., July 26, 2016 (GLOBE NEWSWIRE) -- DeGen Medical has received clearance from the FDA for its F1 MPS™ Modular Pedicle Screw System for JOUST™ Minimally Invasive Surgery (MIS) procedure. The JOUST™ MIS is a modular pedicle screw with cobalt-chrome alloy head for percutaneous procedures.
The JOUST™ MIS technology includes MIS Towers for percutaneous screw placement, traditional or cortical approach, and rod reduction is built in the Tower. The unique Rod Insertion Tool with JOUST™ Minimally Invasive Rods aids in achieving a stable fusion construct without undergoing the traditional open approach. JOUST™ MIS procedure allows the surgeon to place the pedicle screws with minimal muscle stripping causing reduced muscle damage and lessening scar tissue formation from decreased dissection. The robust instrumentation allows for smooth implantation in a complex lumbar spine surgery over multiple levels.
The JOUST™ MIS technology with F1 MPS™ System will be available in Q4 of 2016.
About DeGen Medical, Inc.
DeGen Medical, Inc. is a medical-device development company dedicated to providing surgeons with innovative products engineered to improve quality of life for patients with complex spinal disorders. World-class implants, coupled with intuitively-designed instrumentation, provide a complete package to promote superior surgical outcomes. Our passion to advance spine-care solutions is driven by clinical insights, sound research, and science-based design. DeGen Medical maintains the highest quality standards to provide reliable products and safeguard patient health.
A photo accompanying this release is available at: http://www.globenewswire.com/newsroom/prs/?pkgid=40928