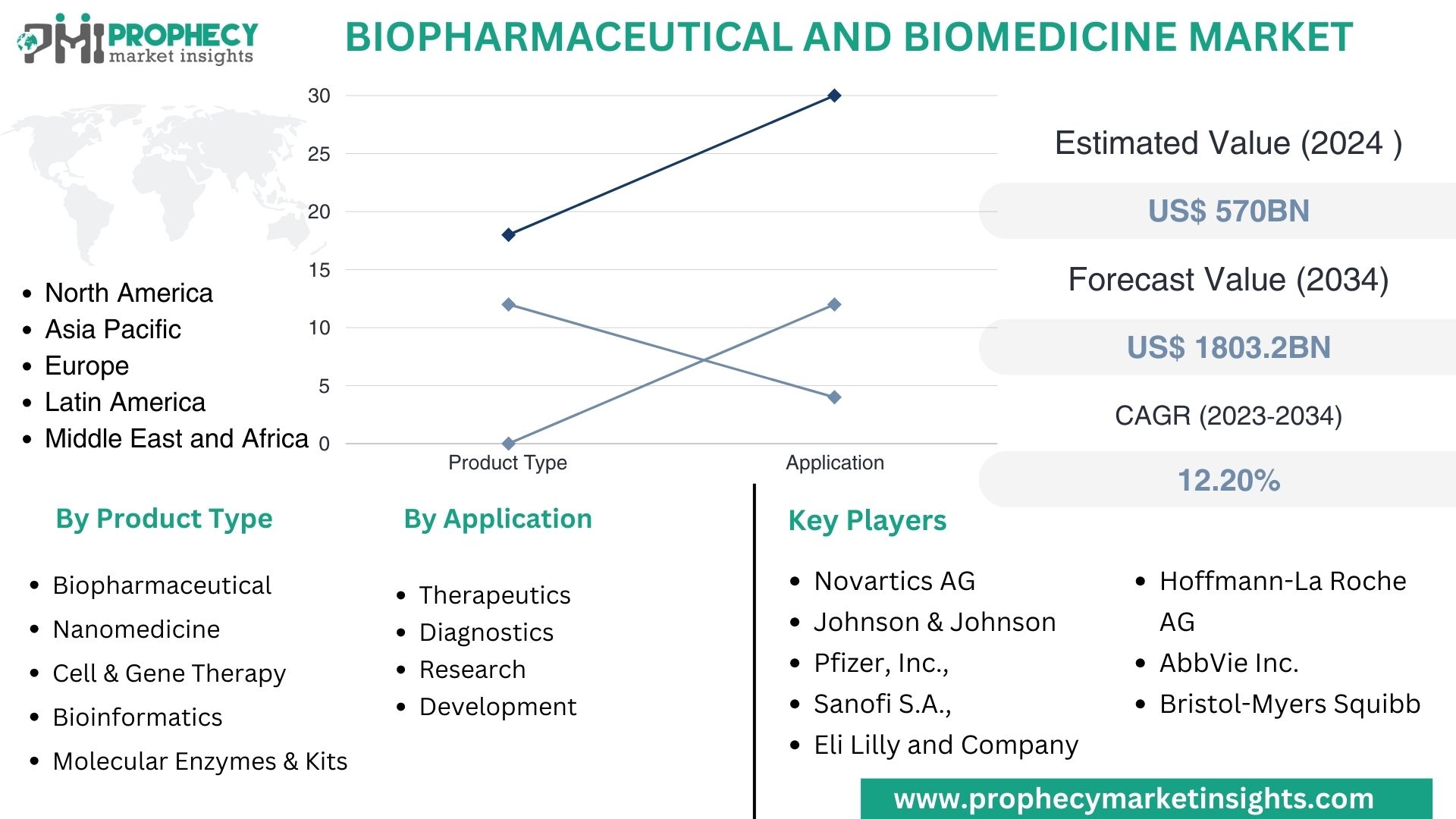
Covina, Feb. 12, 2024 (GLOBE NEWSWIRE) -- “According to the recent research study, the Biopharmaceutical and Biomedicine Market size was valued at about USD 570.0 Billion in 2024 and expected to grow at CAGR of 12.20% to extend a value of USD 1803.2 Billion by 2034.”
What is Biopharmaceutical and Biomedicine?
- Market Overview:
Biopharmaceuticals and biomedicine are two closely related fields within the broader domain of medicine and pharmaceuticals, both of which involve the study, development, and application of biological-based products for medical purposes. While they share similarities, they serve distinct roles in the healthcare industry. Biopharmaceuticals, also known as biologics, are pharmaceutical drugs that are produced using biotechnology processes and techniques. These drugs are derived from living organisms or their components, such as proteins, nucleic acids, antibodies, and other biomolecules.
Biomedicine refers to the interdisciplinary field of medical science that combines principles of biology, physiology, genetics, pharmacology, and other life sciences to understand the mechanisms of disease and develop therapeutic interventions. Biomedicine encompasses a broad range of disciplines and research areas, including molecular biology, genomics, pharmacogenomics, regenerative medicine, and translational research.
Get Access to Free Sample Research Report with Latest Industry Insights:
https://www.prophecymarketinsights.com/market_insight/Insight/request-sample/116
*Note: PMI Sample Report includes,
- Overview & introduction of market study
- Revenue and CAGR of market
- Drivers & Restrains factors of market
- Major key players in market
- Regional analysis of the market with a detailed graph
- Detailed segmentation in tabular form of market
- Recent development/news of market
- Opportunities & Challenges of Market
Top Leading Players in Biopharmaceutical and Biomedicine Market:
- Novartics AG
- Johnson & Johnson
- Pfizer, Inc.
- Sanofi S.A.
- Eli Lilly and Company
- Hoffmann-La Roche AG
- AbbVie Inc.
- Bristol-Myers Squibb
- Qiagen N.V.
- Affimed N.V.
- Celgene Corporation.
Market Dynamics:
Driving Factors:
- Technological advancements in biotechnology, including genomics, proteomics, recombinant DNA technology, and cell-based therapies, drive innovation in biopharmaceutical and biomedicine development, enabling the production of novel therapeutics and diagnostics for various diseases.
- The shift towards personalized medicine approaches, which emphasize patient-specific treatment strategies based on genetic, molecular, and clinical characteristics, fuels demand for biopharmaceuticals and biomedicines tailored to individual patient needs and disease profiles.
- The increasing prevalence of chronic diseases, infectious diseases, cancer, and autoimmune disorders worldwide creates a significant unmet medical need for effective therapies and vaccines, driving investment in biopharmaceutical research and development.
- Emerging economies with growing healthcare infrastructure, rising disposable incomes, and expanding patient populations present lucrative opportunities for biopharmaceutical and biomedicine companies to expand market reach, penetrate new geographic regions, and address unmet medical needs.
Restrain Factors:
- High Development Costs
- Regulatory Complexity
- Manufacturing Complexity and Capacity Constraints
Emerging Trends and Opportunities in Biopharmaceutical and Biomedicine Market:
- The shift towards precision medicine and personalized therapies is transforming the biopharmaceutical and biomedicine market. Advances in genomics, biomarker identification, and molecular diagnostics enable tailored treatment regimens based on individual patient characteristics, genetic profiles, and disease signatures. The development of targeted therapies and companion diagnostics enhances treatment efficacy, minimizes adverse effects, and improves patient outcomes.
- Biological therapies and immunotherapies represent a rapidly growing segment of the biopharmaceutical market, driven by breakthroughs in immunology, cellular biology, and cancer research. Monoclonal antibodies, immune checkpoint inhibitors, CAR-T cell therapies, and other biologics offer novel treatment options for cancer, autoimmune diseases, and inflammatory disorders. Emerging technologies such as gene editing and cell engineering hold promise for the development of next-generation immunotherapies with enhanced specificity and potency.
- Regenerative medicine and stem cell therapies hold immense potential for treating degenerative diseases, tissue injuries, and organ failure. Advances in stem cell biology, tissue engineering, and biomaterials enable the development of innovative therapies for conditions such as spinal cord injury, heart disease, diabetes, and neurodegenerative disorders. The emergence of induced pluripotent stem cells (iPSCs) and organoid models offers new avenues for disease modeling, drug discovery, and personalized medicine applications.
- The biopharmaceutical manufacturing sector is experiencing rapid growth and innovation driven by increasing demand for biologics, biosimilars, and cell-based therapies. Advances in bioprocessing technologies, including continuous manufacturing, single-use systems, and process intensification, optimize production efficiency, reduce costs, and accelerate time to market for biopharmaceutical products. Outsourcing of manufacturing operations and contract development and manufacturing organizations (CDMOs) play a crucial role in scaling up production capacity and ensuring supply chain resilience.
- Digital health technologies and data analytics are revolutionizing healthcare delivery, patient monitoring, and therapeutic development in the biopharmaceutical and biomedicine market. The integration of electronic health records (EHRs), wearable devices, telemedicine platforms, and artificial intelligence (AI) algorithms enables real-time patient monitoring, personalized treatment algorithms, and predictive analytics for disease management and drug development. Data-driven approaches facilitate clinical trial optimization, patient recruitment, and regulatory decision-making, driving efficiency and innovation across the healthcare ecosystem.
Download PDF Brochure:
https://www.prophecymarketinsights.com/market_insight/Insight/request-pdf/116
Challenges of Biopharmaceutical and Biomedicine Market:
- Market access and reimbursement policies play a pivotal role in determining product adoption, pricing, and profitability in the biopharmaceutical and biomedicine market. Limited reimbursement coverage, pricing pressures, and healthcare budget constraints pose challenges for market entry and commercialization, particularly for novel therapies with high development costs and uncertain clinical outcomes. Demonstrating value-based outcomes, cost-effectiveness, and real-world evidence is crucial for securing reimbursement and market acceptance.
- Biopharmaceutical manufacturing facilities and research laboratories are subject to biosafety and biosecurity regulations to prevent the risk of contamination, cross-contamination, and accidental release of biological agents. Ensuring adherence to biosafety protocols, implementing containment measures, and mitigating biosecurity risks are essential for protecting public health, environmental safety, and maintaining public trust in biopharmaceutical products and research activities.
- Ethical and societal considerations surrounding biopharmaceuticals and biomedicine raise complex ethical dilemmas, including issues related to patient consent, genetic privacy, human embryo research, and equitable access to healthcare. Balancing scientific progress with ethical principles, cultural values, and social justice requires transparent dialogue, stakeholder engagement, and ethical frameworks that promote responsible innovation and equitable healthcare delivery.
Detailed Segmentation:
Biopharmaceutical and Biomedicine Market, By Product Type:
-
-
- Biopharmaceutical
- Nanomedicine
- Cell & Gene Therapy
- Bioinformatics
- Molecular Enzymes
- Kits
-
Biopharmaceutical and Biomedicine Market, By Application:
-
-
- Therapeutics
- Diagnostics
- Research And Development
-
Biopharmaceutical and Biomedicine Market, By Region:
-
-
- North America
-
- U.S.
- Canada
-
- Europe
-
- Germany
- UK
- France
- Russia
- Italy
- Rest of Europe
-
- Asia Pacific
-
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
-
- Latin America
-
- Brazil
- Mexico
- Rest of Latin America
-
- Middle East & Africa
-
- GCC
- Israel
- South Africa
- Rest of Middle East & Africa
-
- North America
-
Regional Analysis:
Regional insights highlight the diverse market dynamics, regulatory landscapes, and growth drivers shaping the Biopharmaceutical and Biomedicine Market across different geographic areas. Understanding regional nuances and market trends is essential for stakeholders to capitalize on emerging opportunities and drive market expansion in the Biopharmaceutical and Biomedicine sector.
North America is estimated to witness a huge market growth as, in North America, particularly the United States, serves as a global innovation hub for biopharmaceutical research and development. The region is home to world-leading academic institutions, research organizations, biotechnology companies, and pharmaceutical giants engaged in cutting-edge research, drug discovery, and therapeutic innovation. The presence of robust intellectual property protections, favorable regulatory environment, and access to venture capital and funding opportunities foster a culture of innovation and entrepreneurship in the biopharmaceutical sector.
Report scope:
| Attribute | Details |
| Market Size 2024 | US$ 570.0 billion |
| Projected Market Size 2034 | US$ 1803.2 billion |
| CAGR Growth Rate | 12.20% |
| Base year for estimation | 2023 |
| Forecast period | 2024 – 2034 |
| Market representation | Revenue in USD Million & CAGR from 2024 to 2034 |
| Market Segmentation | By Type - Biopharmaceutical, nanomedicine, cell & gene therapy, bioinformatics, and molecular enzymes & kits By Application - Therapeutics, diagnostics, and research and development |
| Regional scope | North America - U.S., Canada Europe - UK, Germany, Spain, France, Italy, Russia, Rest of Europe Asia Pacific - Japan, India, China, South Korea, Australia, Rest of Asia-Pacific Latin America - Brazil, Mexico, Argentina, Rest of Latin America Middle East & Africa - South Africa, Saudi Arabia, UAE, Rest of Middle East & Africa |
| Report coverage | Revenue forecast, company share, competitive landscape, growth factors, and trends |
Key highlights of the Biopharmaceutical and Biomedicine Market:
- The biopharmaceutical and biomedicine market is experiencing rapid growth driven by advancements in biotechnology, personalized medicine, and regenerative therapies. The market encompasses a wide range of products, including biologics, gene therapies, cell-based therapies, and medical devices, which address diverse medical needs and therapeutic indications.
- The market is characterized by a high level of innovation and research activity, with significant investments in basic science, translational research, and clinical development. Advances in genomics, proteomics, bioinformatics, and molecular biology are driving the discovery and development of novel therapeutic targets, biomarkers, and precision medicine approaches.
- The therapeutic landscape of biopharmaceuticals and biomedicine is expanding rapidly, with new treatments and interventions emerging for a wide range of diseases and medical conditions. Therapeutic areas such as oncology, immunology, neurology, cardiovascular disease, and rare genetic disorders are witnessing significant advancements in treatment options and patient outcomes.
- Personalized medicine approaches, driven by advances in genomic sequencing, molecular diagnostics, and targeted therapies, are revolutionizing the way diseases are diagnosed, treated, and managed. Precision medicine strategies enable tailored treatment regimens based on individual patient characteristics, genetic profiles, and disease signatures, leading to improved therapeutic outcomes and patient care.
- Biopharmaceutical manufacturing is evolving to meet the growing demand for biologics and complex therapies. Advances in bioprocessing technologies, including cell culture systems, purification techniques, and continuous manufacturing platforms, enhance production efficiency, reduce costs, and improve product quality and consistency.
- Regulatory agencies play a critical role in ensuring the safety, efficacy, and quality of biopharmaceutical and biomedicine products. Stringent regulatory standards, including Good Manufacturing Practices (GMP), Good Clinical Practice (GCP), and regulatory approval processes, ensure compliance with regulatory requirements and promote public health and patient safety.
Any query or customization before buying:
https://www.prophecymarketinsights.com/market_insight/Insight/request-customization/116
Explore More Insights:
- Biologics CDMO Market- Trends, Analysis and Forecast till 2034
- Cosmetic Peptide Synthesis Market– Trends, Analysis and Forecast till 2034
- Induced Pluripotent Stem Cells Market - Trends, Analysis and Forecast till 2034
Blog: www.prophecyjournals.com
Follow us on:
LinkedIn | Twitter | Facebook |YouTube
