Dublin, April 09, 2024 (GLOBE NEWSWIRE) -- The "Lyophilization Services for Biopharmaceuticals Global Market Report 2024" report has been added to ResearchAndMarkets.com's offering.
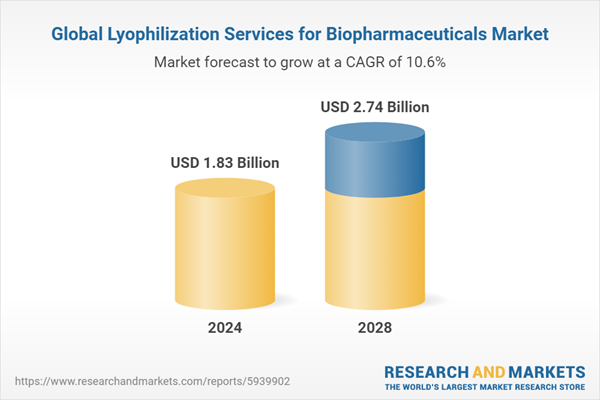
The lyophilization services for biopharmaceuticals market size has grown rapidly in recent years. It will grow from $1.66 billion in 2023 to $1.83 billion in 2024 at a compound annual growth rate (CAGR) of 10.5%. The expansion observed in the historical period can be attributed to several factors, including the growth of the biopharmaceutical sector, the expiration of drug patents, the surge in personalized medicine adoption, an increase in clinical trials, and the globalization of the biopharmaceutical market.
The market size is expected to see continued growth in the next few years, reaching $2.74 billion in 2028 at a CAGR of 10.6%. The anticipated growth in the forecast period can be linked to various factors, including the emergence of mRNA and nucleic acid therapeutics, outsourcing trends within the pharmaceutical industry, a heightened emphasis on continuous manufacturing, advancements in combination therapies, and the expanding production of biosimilars. Notable trends expected during this period encompass the implementation of Quality-by-Design (QbD), integration of automation and robotics, a heightened focus on sustainability, customization tailored for complex molecules, and a notable shift towards continuous lyophilization.
The lyophilization services for the biopharmaceuticals market are anticipated to experience substantial growth due to the increasing demand for biologics. Biologics, which are therapeutic products derived from living organisms, or containing components of living organisms, are prominently utilized in the pharmaceutical and biopharmaceutical industries. Lyophilization services play a crucial role in preserving the stability of biologics by preventing denaturation or degradation, thereby enhancing stability and improving product integrity. Notably, reports from the Canadian Institute for Health Information reveal that biologics accounted for a significant portion of total spending on public medication programs in 2021, underlining the growing importance of these products. Additionally, the US Food and Drug Administration's approval of numerous biosimilars underscores the increasing competition and demand for biologics in the United States, further fueling the need for lyophilization services in the biopharmaceuticals market.
The growth of disposable incomes is expected to be a key driver for the lyophilization services market in the biopharmaceutical sector. Rising disposable incomes empower individuals and healthcare systems to invest in advanced medical treatments, including biopharmaceuticals. This increased demand contributes to heightened investments in the development and manufacturing of these products, with a specific focus on specialized services such as lyophilization. For instance, data from the Statistics Bureau of Japan and the U.S. Bureau of Economic Analysis reveal substantial increases in disposable personal income in Japan and the United States, respectively. This upward trend in disposable incomes is a significant factor propelling the growth of lyophilization services in the biopharmaceuticals market.
The lyophilization services for biopharmaceuticals market is witnessing a notable trend towards technological advancements, with major companies focusing on developing innovative specialized products to enhance their market standing. An example of this trend is evident in the launch of the SP Hull LyoStar 4.0 R&D freeze dryer by SP Industries Inc., a prominent US-based pharmaceutical product manufacturer. This pilot-scale freeze dryer, introduced in June 2021, is designed to expedite the market for biopharmaceutical products. Leveraging features such as superior shelf mapping, rapid shelf freezing, unmatched process accuracy, and reliability, LyoStar 4.0 is based on a full-scale production freeze dryer, supporting rapid scale-up. It incorporates an innovative suite of Process Analytical Technology (PAT) tools, providing real-time data on the lyophilization process. This technology ensures precise process control, robust reliability, and protection of valuable products, potentially reducing the time and cost associated with developing new biopharmaceutical products while improving their quality and consistency.
The key categories within lyophilization services for biopharmaceuticals include product and cycle development, clinical manufacturing, commercial manufacturing, and freeze-drying analytical services. Product and cycle development encompass the process of designing, developing, and optimizing a product along with its manufacturing cycle. This includes the utilization of various packaging systems, such as vials, syringes, cartridges, ampoules, and others. These services cater to diverse applications, including pharmaceutical and biotechnology companies, research institutes, and other relevant industries.
The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market's historic and forecast market growth by geography.
Markets Covered:
- By Type: Product and Cycle Development; Clinical Manufacturing; Commercial Manufacturing; Freeze Drying Analytical Services
- By Type Of Primary Packaging System: Vials; Syringes; Cartridges; Ampoules; Other Primary Packaging Systems
- By Application: Pharmaceutical and Biotechnology Companies; Research Institutes; Other Applications
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 175 |
| Forecast Period | 2024 - 2028 |
| Estimated Market Value (USD) in 2024 | $1.83 Billion |
| Forecasted Market Value (USD) by 2028 | $2.74 Billion |
| Compound Annual Growth Rate | 10.6% |
| Regions Covered | Global |
Companies Profiled:
- Strides Pharma Science
- Baxter International
- Catalent Pharma Solutions
- W. L. Gore & Associates
- Emergent BioSolutions
- Vetter Pharma International
- Curia Global
- Corden Pharma
- CinnaGen
- Lyophilization Services of New England
- Jubilant HollisterStier
- Dalton Pharma Services
- IDT Biologika
- Ardena Group
- Axcellerate Pharma
- Symbiosis Pharmaceutical Services
- Lyka Labs Limited
- Biofortuna Limited
- Rentschler Fill Solutions
- Lyophilization Technology
- Pyramid Laboratories
- ATTWILL Medical Solutions
- AB BioTechnologies Inc.
- Biopharma Process Systems
- Labyrinth Biopharma
- Gilyos GmbH
- ProJect Pharmaceutics
- BioZed Engineering
- Berkshire Sterile Manufacturing
- Millrock Technology
For more information about this report visit https://www.researchandmarkets.com/r/erdslt
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
