Study demonstrates strong statistical separation between patients receiving Nexalin’s DIFS® technology in combination with escitalopram (Lexapro) vs sham group receiving escitalopram alone
Two-thirds of patients receiving the combination therapy showed improvement
compared to just one-third of patients receiving medication alone
HOUSTON, June 26, 2024 (GLOBE NEWSWIRE) -- Nexalin Technology, Inc. (Nasdaq: NXL; NXLIW) (the “Company” or “Nexalin”) today announced positive results of a clinical study designed to evaluate the feasibility, safety, and efficacy of transcranial alternating current dynamic frequency stimulation (tACS) as an add-on treatment for the symptoms of major depressive disorder (MDD), also known as clinical depression, a mental health condition that affects mood, behavior, appetite, and sleep.
The study was designed as a 4-week, double-blind, randomized, sham-controlled trial, in which sixty-six participants were recruited and randomly assigned to receive 20 40-minute sessions of either active treatment using the Company’s non-invasive Deep Intracranial Frequency Stimulation (DIFS®) technology (77.5Hz, 15 mA) or sham stimulation. The study involved the combined use of escitalopram (Lexapro), a selective serotonin reuptake inhibitor (SSRI), throughout the 4-week period. Escitalopram is a commonly prescribed medication used to treat anxiety and major depressive disorder.
Significant differences were found in the reductions in the Hamilton Depression Rating Scale (HAMD-17) scores at week 4 (t = 3.44, P = 0.001). Response rates at week 4 were significantly higher in the active tACS group than in the sham tACS group (22 out of 33 patients [66.7 %] versus 11 out of 33 [33.3 %], P = 0.007). In the active tACS group, a correlation between the mean change in alpha power and HAMD-17 scores at week 4 was found (r = 2.38, P = 0.024), and the mean change in alpha power was significantly greater for responders (Z = 2.46, P = 0.014). No serious adverse events were observed in this trial.
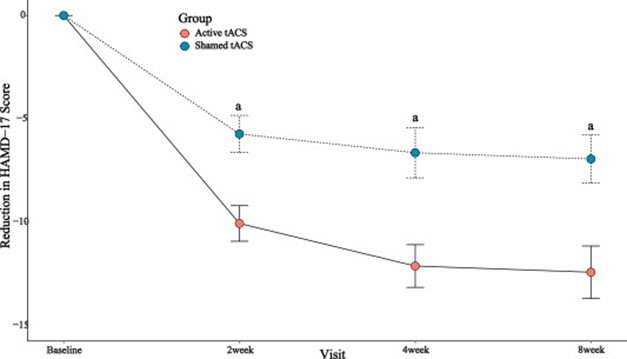
The results of the study were published in Brain Stimulation, the premier peer-reviewed journal in the field of neuromodulation. The article, titled “Effect of add-on transcranial alternating current stimulation (tACS) in major depressive disorder: A randomized controlled trial,” concluded: “The additional antidepressant effect of tACS is significant, and the combination of tACS with antidepressants is a feasible and effective approach for the treatment of MDD.”
Dr. David Owens, Chief Medical Officer of Nexalin, commented, "We are proud to announce these positive results and are honored to have the results published in such a prominent, peer-reviewed journal. While we have previously demonstrated powerful treatment effects from our DIFS technology alone, this was the first study in which we evaluated our technology in combination with an SSRI, which has become the gold standard treatment in the U.S. Most notably, we believe that this study demonstrated strong and statistically significant separation between the active group receiving treatment with DIFS and medication, versus the placebo groups receiving medication alone—exactly the results we were hoping to demonstrate in the trial. In fact, only one-third of the treated patients receiving medication alone showed improvement, whereas two-thirds receiving the combination showed improvement. Also noteworthy was the fact that no significant adverse effects were reported.”
Mark White, CEO of Nexalin, stated, “We believe this data strongly reinforces the growing body of clinical evidence supporting the potential of Nexalin's new advanced 15 mAmp waveform to help combat the ongoing global mental health epidemic—both as a standalone drug-free treatment alternative, or in combination with existing pharmacological therapies. According to a 2022 National Survey on Drug Use and Health, an estimated 22.47 million adults, or 8.8% of all US adults in the United States, had at least one major depressive episode. Moreover, according to Future Market Insights, the MDD treatment market is forecast to reach US$ 14.96 billion by 2032 from US$ 11.51 billion in 2022. We believe the data provides further evidence of the significant impact of our non-invasive drug-free device on improving mental healthcare outcomes among patients affected with MDD. We look forward to advancing our technology in order to bring our new, effective therapies to the millions of patients suffering from mental health issues in the United States and around the world.”
About Nexalin Technology, Inc.
Nexalin designs and develops innovative neurostimulation products to uniquely help combat the ongoing global mental health epidemic. All of Nexalin’s products are believed to be non-invasive and undetectable to the human body and are developed to provide relief to those afflicted with mental health issues. Nexalin utilizes bioelectronic medical technology to treat mental health issues. Nexalin believes its neurostimulation medical devices can penetrate structures deep in the mid-brain that are associated with mental health disorders. Nexalin believes the deeper-penetrating waveform in its next-generation devices will generate enhanced patient response without any adverse side effects. The Nexalin Gen-2 15 milliamp neurostimulation device has been approved in China, Brazil, and Oman. Additional information about the Company is available at: https://nexalin.com/.
FORWARD-LOOKING STATEMENTS
This press release contains statements that constitute "forward-looking statements," These statements relate to future events or Nexalin’s future financial performance. Any statements that refer to expectations, projections or other characterizations of future events or circumstances or that are not statements of historical fact (including without limitation statements to the effect that Nexalin or its management “believes”, “expects”, “anticipates”, “plans”, “intends” and similar expressions) should be considered forward looking statements that involve risks and uncertainties which could cause actual events or Nexalin’s actual results to differ materially from those indicated by the forward-looking statements. Forward-looking statements are subject to numerous conditions, many of which are beyond the control of the Company, including those set forth in the Risk Factors section of the Company's Report on Form 10-K for the year ended December 31, 2023 and other filings as filed with the Securities and Exchange Commission. Copies of such filings are available on the SEC's website, www.sec.gov. Such forward-looking statements are made as of the date hereof and may become outdated over time. The Company undertakes no obligation to update these statements for revisions or changes after the date of this release, except as required by law.
Contact:
Crescendo Communications, LLC
Tel: (212) 671-1020
Email: NXL@crescendo-ir.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ca5f1a24-dc0a-4bb1-9bd1-86203176a408
