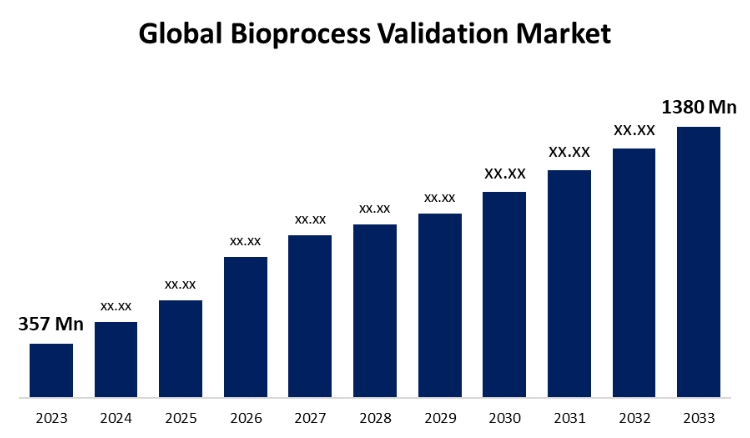
New York, United States , June 28, 2024 (GLOBE NEWSWIRE) -- The Global Bioprocess Validation Market Size is to Grow from USD 357 Million in 2023 to USD 1380 Million by 2033, at a Compound Annual Growth Rate (CAGR) of 14.48% during the projected period.
Get a Sample PDF Brochure: https://www.sphericalinsights.com/request-sample/4500
Bioprocess validation is the process of documenting each stage, action, and piece of evidence utilized in the production of biological and biopharmaceutical products. The documentation is completed by US FDA guidelines and current rules governing product manufacturing processes. It guarantees compliance for the duration of the product testing procedure. Bioprocess validation requires an assessment of the active pharmaceutical ingredients as well as specific contaminants including bacteria, mycoplasma, and endotoxins. More specifically, data is first collected, assessed, and then documented from each stage of a project at all crucial times. Bioprocess validation offers concrete evidence in this way that it consistently produces high-quality products. The increasing demand for bioprocess validation, a practice that ensures the safety, sustainability, and efficacy of biopharmaceutical products and processes, is emphasized by the global bioprocess validation market. Outsourcing bioprocess validation market services is particularly sought after, as it fulfills regulatory criteria in the healthcare industry and ensures product manufacturing practice compliance. Furthermore, there are errors in the testing protocols and validation process phases in bioprocess validation. Internal sources or individuals performing the validation process can make mistakes. The samples can include false positives or negatives, which could alter the result.
Browse key industry insights spread across 220 pages with 110 Market data tables and figures & charts from the report on the "Global Bioprocess Validation Market Size, Share, and COVID-19 Impact Analysis, By Test Type (Extractable and Leachable Testing, Integrity Testing, Bioprocess Residuals Testing, Viral Clearance Testing, Systems Testing, Compatibility Testing, Microbiological Testing, Physiochemical Testing, Bacterial Retention Testing, and Others), By Process Component (Filter Elements, Media Containers And Bags, Bioreactors, Freezing And Thawing Process Bags, Mixing Systems, Tubing, Connectors, Samplers, And Others), By End User (Pharmaceutical Companies, Biotechnology Companies, Contract Development & Manufacturing Organizations, and Others), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033."
Buy Now Full Report https://www.sphericalinsights.com/checkout/4500
The extractable and leachable testing segment dominates the market with the largest market share of the global bioprocess validation market during the projected timeframe.
The global bioprocess validation market is classified by test type into extractable and leachable testing, integrity testing, bioprocess residuals testing, viral clearance testing, systems testing, compatibility testing, microbiological testing, physiochemical testing, bacterial retention testing, and others. Among these, the extractable and leachable testing segment dominates the market with the largest market share of the global bioprocess validation market during the projected timeframe. Testing using extractable and leachable materials is done to find potentially hazardous substances that could be given to the patient together with the treatment or drug. The goal of extractable and leachable testing studies is to determine whether potentially harmful organic and inorganic impurities affect the safety or efficacy of the finished product.
The filter elements segment is anticipated to grow at the fastest pace in the global bioprocess validation market during the projected timeframe.
The global bioprocess validation market is classified by process component into filter elements, media containers and bags, bioreactors, freezing and thawing process bags, mixing systems, tubing, connectors, samplers, and others. Among these, the filter elements segment is anticipated to grow at the fastest pace in the global bioprocess validation market during the projected timeframe. The components of the filter in the bioprocess validation process, ensuring the integrity of the products is an essential stage, with the filter elements serving as the foundation. They remove all unwanted particles, including bacteria, and create high-quality products.
The pharmaceutical companies segment accounted for the largest revenue share in the bioprocess validation market during the estimated period.
On the basis of end user, the bioprocess validation market is differentiated into biotechnology companies, pharmaceutical companies contract development & manufacturing organizations, and others. Among these, the pharmaceutical companies segment accounted for the largest revenue share in the bioprocess validation market during the estimated period. Pharmaceutical companies conduct research and development and manufacture and supply drugs, vaccines, and diagnostic tests to patients to treat, prevent, or diagnose illnesses and ailments. The growth of biopharmaceutical manufacturing and the corresponding rise in contaminants that require testing, along with strict laws and rules controlling the quality and dependability of the bioprocesses utilized in their development, are the primary causes of pharmaceutical firms.
Inquire Before Buying This Research Report: https://www.sphericalinsights.com/inquiry-before-buying/4500
Asia Pacific is expected to hold the largest share of the global bioprocess validation market over the forecast period.
Asia Pacific is expected to hold the largest share of the global bioprocess validation market over the forecast period. This is a result of rising healthcare costs, government-established R&D money, and technology developments. A growing understanding of the advantages and benefits of vaccines and biopharmaceutical medications for the treatment of chronic diseases supports government initiatives for the expansion and development of the biopharmaceutical industry in the Asia Pacific region. Growing life science research on biologics, rising demand for bioprocess validation outsourcing, growing biopharmaceutical manufacturing capabilities in Asian countries, and rising investments from biotechnology and pharmaceutical companies are expected to propel the market expansion.
North America is predicted to grow at the fastest pace in the global bioprocess validation market during the projected timeframe. This is a result of substantial outsourcing services being provided. Biologics output rises as a result, and life science research grows, propelling the global market.
Competitive Analysis:
The report offers the appropriate analysis of the key organizations/companies involved within the global market along with a comparative evaluation primarily based on their product offering, business overviews, geographic presence, enterprise strategies, segment market share, and SWOT analysis. The report also provides an elaborative analysis focusing on the current news and developments of the companies, which includes product development, innovations, joint ventures, partnerships, mergers & acquisitions, strategic alliances, and others. This allows for the evaluation of the overall competition within the market. Major vendors in the global bioprocess validation market include Eurofins Scientific, Inc., Thermo Fisher Scientific Inc., Biozeen, Pall Corporation, Danaher Corporation, Sartorius Stedium Biotech, Porvair Plc, LabCorp Corporation, Cobetter Filtration equipment Co., Ltd., Corning Inc.,Toxikon Corporation, Charles River Laboratories, Cytovance, Lonz Group, and Others.
Get Discount At @ https://www.sphericalinsights.com/request-discount/4500
Recent Developments
- In February 2022, Thermo Fisher Scientific, Inc and Moderna, Inc. disclosed a 15-year strategic collaboration deal to approve the dedicated large-scale production in the US of Moderna's COVID-19 vaccine, Spikevax®, and other experimental mRNA treatments that are in development.
Market Segment
This study forecasts revenue at global, regional, and country levels from 2020 to 2033. Spherical Insights has segmented the global bioprocess validation market based on the below-mentioned segments:
Global Bioprocess Validation Market, By Test Type
- Extractables and Leachables Testing
- Integrity Testing
- Bioprocess Residuals Testing
- Viral Clearance Testing
- Systems Testing
- Compatibility Testing
- Microbiological Testing
- Physiochemical Testing
- Bacterial Retention Testing
- Adsorption Testing
- Others
Global Bioprocess Validation Market, By Process Component
- Filter elements
- Media containers and bags
- Bioreactors
- Freezing and thawing process bags
- Mixing systems
- Tubing
- Connectors
- Samplers
- Others
Global Bioprocess Validation Market, By End User
- Pharmaceutical Companies
- Biotechnology Companies
- Contract Development & Manufacturing Organizations
- Others
Global Bioprocess Validation Market, By Regional
- North America
- US
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Russia
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia Pacific
- South America
- Brazil
- Argentina
- Rest of South America
- Middle East & Africa
- UAE
- Saudi Arabia
- Qatar
- South Africa
- Rest of the Middle East & Africa
Browse Related Reports
Global Nucleotides Market Size, Share, and COVID-19 Impact Analysis, By Nitrogenous Base (Purine and Pyrimidine), By Application (Diagnostics Research, Pharmaceuticals, and Others), By Technology (TaqMan Allelic Discrimination and SNP By Pyrosequencing), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 – 2033
Global Skin Rash Treatment Market Size, Share, and COVID-19 Impact Analysis, By Skin Rash Type (Contact Dermatitis, Eczema, Psoriasis, Hives, Viral, and Others), By Treatment Type (Corticosteroids, Immunosuppressants, Antihistamines, Antifungals, and Others), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Online Pharmacies), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033.
Global Nasal Spray Market Size, Share, and COVID-19 Impact Analysis, By Product Type (Corticosteroids, salt water solutions, Topical Decongestants, Antihistamine, and Others), By Application (Nasal Allergies, Cold Asthma, and Others), By Age Group (Pediatric and Adults), By Distribution Channel (Drug Stores & Retail Pharmacies, Hospital Pharmacies, and Online Pharmacies), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
Global Insulin Glargine Market Size, Share, and COVID-19 Impact Analysis, By Type (Lantus, Basaglar, Toujeo, Soliqua, Rezvoglar, and Others), By Diabetes Type (Type 2 Diabetes, Type 1 Diabetes), By Distribution Channel (Hospital Pharmacies, Online Pharmacies and Retail Pharmacies), and By Region (North America, Europe, Asia-Pacific, Latin America, Middle East, and Africa), Analysis and Forecast 2023 - 2033
About the Spherical Insights & Consulting
Spherical Insights & Consulting is a market research and consulting firm which provides actionable market research study, quantitative forecasting and trends analysis provides forward-looking insight especially designed for decision makers and aids ROI.
Which is catering to different industry such as financial sectors, industrial sectors, government organizations, universities, non-profits and corporations. The company's mission is to work with businesses to achieve business objectives and maintain strategic improvements.
CONTACT US:
For More Information on Your Target Market, Please Contact Us Below:
Phone: +1 303 800 4326 (the U.S.)
Phone: +91 90289 24100 (APAC)
Email: inquiry@sphericalinsights.com, sales@sphericalinsights.com
Contact Us: https://www.sphericalinsights.com/contact-us
Follow Us: LinkedIn | Facebook | Twitter
