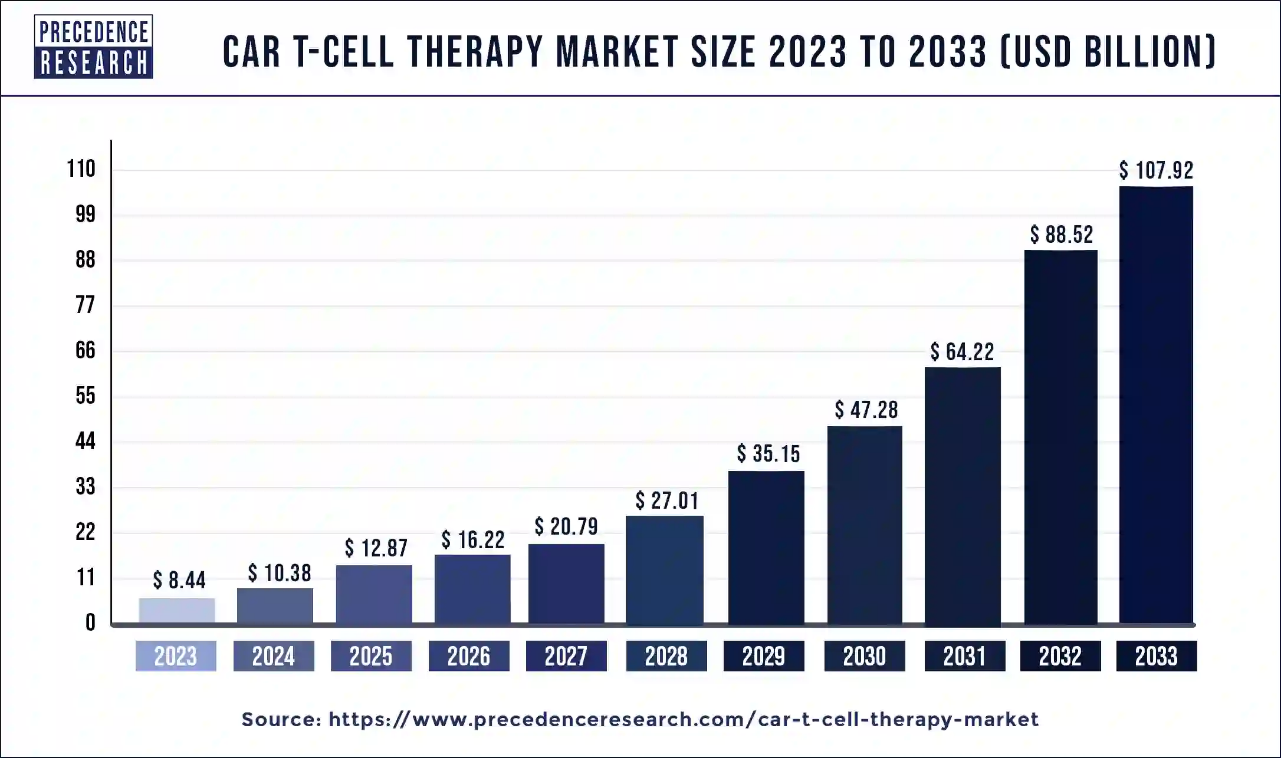
Ottawa, July 01, 2024 (GLOBE NEWSWIRE) -- The global CAR T-cell therapy market size is predicted to increase from USD 8.44 billion in 2023 to approximately USD 107.92 billion by 2033, According to Precedence Research. The industry is expected to grow at a remarkable CAGR of 30% during the forecast period. The CAR T-cell therapy market is driven by increasing cancer prevalence and evolving technologies.
The CAR T-cell therapy market encompasses the biopharmaceutical industry segment that is focused on the development, production, and commercialization of chimeric antigen receptor (CAR) T-cell therapies. Chimeric antigen receptor (CAR) T cells are fusion proteins that direct T cells to antigens on tumor cells, resulting in an antitumor immune response. Over a decade ago, CAR T cells that target CD19, which is expressed in malignant B cells, demonstrated substantial efficiency in clinical studies involving patients with relapsed and refractory B cell malignancies.
These cells produced full response rates of 40-54%, 67%, and 96-74% in patients with aggressive B cell lymphomas, indolent B cell lymphoma, and mantle cell lymphoma. CAR T cell treatments, which have been authorized by the FDA for treating R/R aggressive B cell lymphomas, indolent B cell lymphomas, and mantle cell lymphomas, have emerged as an important component of the therapy landscape for a variety of hematological malignancies.
The Full Study is Readily Available | Download the Sample Pages of this Report@ https://www.precedenceresearch.com/sample/2545
CAR T-Cell Therapy Market Revenue (US$ Million), By Drug Type, 2020 to 2023
| Drug Type | 2020 | 2021 | 2022 | 2023 |
| Axicabtagene Ciloleucel | 321.2 | 542.5 | 1,118.3 | 2,472.3 |
| Tisagenlecleucel | 279.8 | 471.9 | 971.1 | 2,143.5 |
| Brexucabtagene Autoleucel | 232.2 | 393.7 | 814.8 | 1,808.5 |
| Others | 270.2 | 452.0 | 922.8 | 2,020.3 |
CAR T-Cell Therapy Market Revenue (US$ Million), By Indication, 2020 to 2023
| Indication | 2020 | 2021 | 2022 | 2023 |
| Lymphoma | 545.7 | 917.1 | 1,881 | 4,137.9 |
| Acute Lymphocytic Leukemia | 415.7 | 703.8 | 1,454.4 | 3,223.1 |
| Others | 142.0 | 239.1 | 491.6 | 1,083.6 |
CAR T-Cell Therapy Market Revenue (US$ Million), By End User, 2020 to 2023
| End User | 2020 | 2021 | 2022 | 2023 |
| Hospitals | 591.7 | 1,003.3 | 2,076.4 | 4,608.8 |
| Cancer Treatment Centers | 511.7 | 856.7 | 1,750.6 | 3,835.8 |
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/2545
CAR T-Cell Therapy Market Key Insights
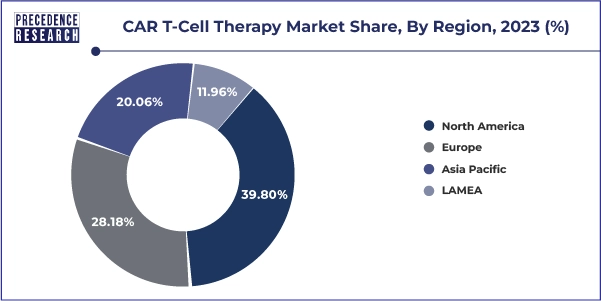
- North America has held the largest revenue share of 39.80% in 2023.
- By drug, the axicabtagene ciloleucel segment has generated the biggest revenue share of 29.28% in 2023.
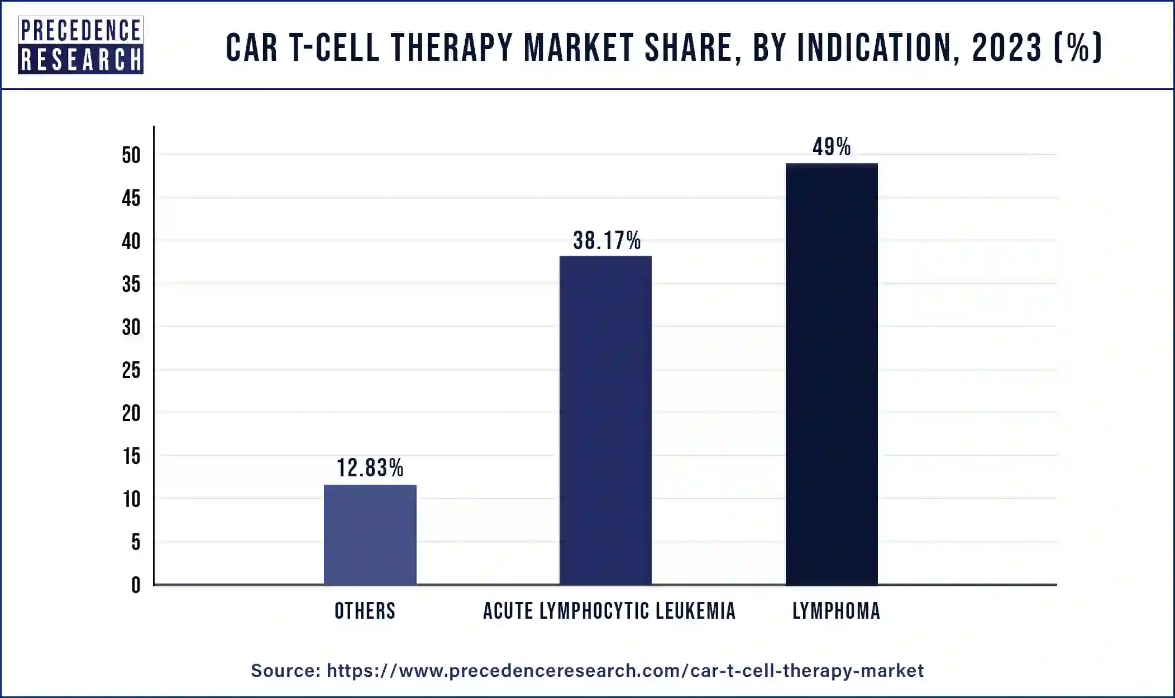
- By indication, the lymphoma segment has recorded more than 49% of revenue share in 2023.
- By end user, the hospital segment has captured 54.58% revenue share in 2023.
CAR T-Cell Therapy Market Projections for Growth by Region Shows:
- North America car t-cell therapy market size was estimated at USD 3.36 billion in 2023 and is expected to reach a CAGR of 30% from 2024 to 2033.
- Europe car t-cell therapy market surpassed at USD 2.37 billion in 2023 and is expected to reach a CAGR of 30% from 2024 to 2033.
- Asia Pacific car t-cell therapy market size was valued at USD 1.69 billion in 2023 and is poised to reach a CAGR of 30% from 2024 to 2033.
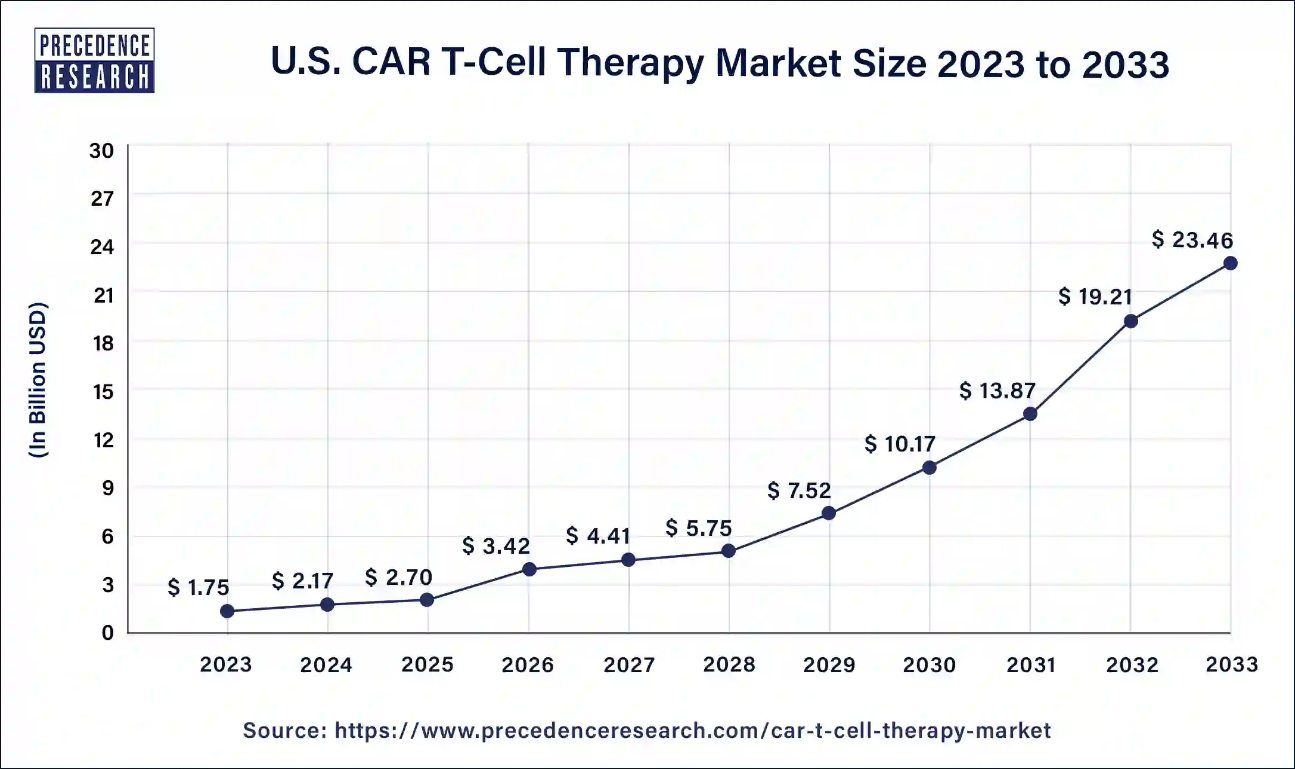
U.S. CAR T-Cell Therapy Market Size and Forecasts
The U.S. car t-cell therapy market size accounted for USD 2.17 billion in 2024 and is estimated to be worth around USD 23.46 billion by 2033, growing at a notable CAGR of 30% from 2024 to 2033.
North America dominated the CAR T-cell therapy market in 2023. Various approvals and product launches are major reasons for market growth. Increasing cases of cancer propel the growth of the market. In 2023, the U.S. was predicted to have 184,720 persons diagnosed with leukemia, lymphoma, or myeloma, accounting for 9.4% of all new cancer cases. These illnesses are estimated to cause 57,380 facilities, accounting for 9.4% of all cancer deaths. The 5-year relative survival rate for leukemia has doubled, with 437,337 patients now in remission. Hodgkin and non-Hodgkin lymphoma have also increased, with a 5-year survival rate of 95.8% for individuals under the age of 50.
Asia Pacific is observed to grow at the fastest rate in the CAR T-cell therapy market. Asia-Pacific countries are actively participating in clinical trials and research studies related to CAR T-cell therapies. This involvement not only advances scientific understanding but also accelerates regulatory approvals for these therapies in the region. Improvements in healthcare infrastructure and increasing access to advanced medical treatments in countries like China, Japan, and South Korea are facilitating the adoption of CAR T-cell therapies. Regulatory bodies in countries such as China are increasingly supportive of innovative therapies like CAR T-cell therapies, streamlining the approval process and facilitating market entry.
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
Scope of CAR T-Cell Therapy Market
| Report Attribute | Key Statistics |
| CAR T-Cell Therapy Market Size by 2033 | USD 107.92 Billion |
| CAR T-Cell Therapy Market Size in 2024 | USD 10.38 Billion |
| CAR T-Cell Therapy Market Size in 2023 | USD 8.44 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 30% |
| Base Year | 2023 |
| Historical Year | 2021-2022 |
| Forecast Year | 2024-2033 |
| Segments Covered | Drug Type, Indication, End User and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
CAR T-Cell Therapy Market Report Highlights
Drug Outlook
The axicabtagene ciloleucel segment dominated the CAR T-cell therapy market in 2023. Axicabtagene ciloleucel is an immunotherapy drug used to treat large B-cell lymphoma in adults. It is administrated after at least two earlier therapies have failed and is manufactured from white blood cells obtained from the patient's blood. It is provided through a specific program, and you must be enrolled. It is only available in accredited hospitals and clinics and must be administered by carefully qualified healthcare experts.
Axicabtagene ciloleucel is administrated during leukapheresis, a technique in which blood cells are collected via a catheter and transferred to a laboratory for processing. Patients are given chemotherapy three to five days before getting the medicine. Other drugs are prescribed to avoid adverse effects or allergic reactions. The drug is then injected into a vein through an IV.
Customize this study as per your requirement@ https://www.precedenceresearch.com/customization/2545
Indication Outlook
The lymphoma segment dominated the CAR T-cell therapy market in 2023. In 2022, the International Agency for research on Cancer (IARC) recorded roughly 20 million new cancer cases and 9.7 million fatalities all over the world. Lung cancer was the most diagnosed cancer, accounting for about 2.5 million new cases and one in every eight malignancies globally. Lung cancer was the largest cause of cancer death, accounting for an estimated 1.8 million fatalities.
End User Outlook
The hospital segment dominated the CAR T-cell therapy market in 2023. Hospitals provide comprehensive medical treatment, screen patients based on their concerns, and deliver short-term care to meet pressing requirements. They collaborate with doctors from a variety of backgrounds to solve medical concerns. While not always receiving expert care, patients might learn about the next stages in their treatment plan. Hospitals are open 24/7, including holidays, and may handle serious ailments with overnight stays, assuring continuous treatment and monitoring by staff personnel.
Browse More Insights:
- Cell Therapy Market Size and Forecast: The global cell therapy market was valued at USD 14.52 billion in 2023 and it is expected to hit USD 97 billion by 2033, poised to grow at a CAGR of 20.9% during the forecast period 2024 to 2033.
- Cancer Biological Therapy Market Size and Forecast: The global cancer biological therapy market size was reached at USD 98.73 billion in 2022 and it is projected to hit around USD 213.6 billion by 2032 with a noteworthy CAGR of 8.02% between 2023 and 2032.
- Drug Device Combination Products Market Size and Forecast: The global drug device combination products market size was USD 139.02 billion in 2023, calculated at USD 151.57 billion in 2024 and is expected to reach around USD 330.02 billion by 2033, expanding at a CAGR of 9.03% from 2024 to 2033.
- Oncology Market Size and Forecast: The global oncology market was valued at US$ 203.42 billion in 2022 and is expected to reach over US$ 470.61 billion by 2032, poised to grow at a noteworthy CAGR of 8.8% from 2023 to 2032.
- Biotechnology Market Size and Forecast: The global biotechnology market size was estimated at USD 1.38 trillion in 2023 and is expected to be worth around USD 4.25 trillion by 2033 and poised to grow at a noteworthy CAGR of 11.8% from 2024 to 2033.
- Telemedicine Market Size and Forecast: The global telemedicine market size was valued at USD 60.8 billion in 2022 and it is expected to reach USD 225 billion by 2030, growing with a compound annual growth rate (CAGR) of 17.16% during the forecast period 2022 to 2030.
- Acute Lymphocytic Leukemia Therapeutics Market Size and Forecast: The global acute lymphocytic leukemia therapeutics market size was reached at USD 3.12 billion in 2022 and is expected to hit around USD 8.29 billion by 2032, growing at a CAGR of 10.25% during the forecast period from 2023 to 2032.
- Dermatology Market Size and Forecast: The global dermatology market size was estimated at USD 1.59 billion in 2022 and it is expected to hit around USD 3.14 billion by 2032, poised to grow at a CAGR of 7.03% during the forecast period from 2023 to 2032.
- Nuclear Medicine Market Size and Forecast: The global nuclear medicine market size accounted for USD 10.65 billion in 2023 and is projected to surpass around USD 31.44 billion by 2033, expected to grow at a CAGR of 11.45% during the forecast period 2024 to 2033.
CAR T-Cell Therapy Market Dynamics
Driver: Increasing cases of cancer
Increasing cancer prevalence is a major driver of the CAR T-cell therapy market. Cancer is a major worldwide health concern, accounting for almost one in every six fatalities and one in every four from noncommunicable diseases (NCDs). It is a serious impediment to improving life expectancy, with significant social and financial implications.
In 2022, GLOBOCAN projections showed the worldwide cancer burden, with 36 cancer types and an emphasis on the top ten. The study also looked at regional heterogeneity in 20 selected global areas and estimated the future cancer burden in 2050 using global population estimates. The study also assessed the burden using the human development index IHDI) and predicted the future burden of cancer in 2050.
Restraint: Antigen escape
CAR T-cell therapy poses hurdles when tumors acquire resistance to single antigen-targeting CAR designs. This condition, known as antigen escape, occurs even when malignant cells in many patients exhibit partial to total loss of target antigen expression.
Despite long-lasting responses in relapsed and refractory ALL patients, new follow-up data indicate a shared disease resistance mechanism, including downregulation/loss of CD19 antigen in 30-70% of individuals with recurrent illness following therapy. Strategies for tandem CARs. Preliminary clinical trial outcomes utilizing dual-targeted CAR-T cells have been promising, with greater anti-tumor responses compared to single-target treatment.
Opportunity: New technology for improving therapy efficiency
Multiple myeloma therapy options are limited, although CAR T-cells, modified T cells with lymphocyte-like signaling molecules, have shown promise in targeting genes in malignant cells. Clinical trials are currently being conducted to investigate combinations of medicines, but identifying effective targets and appropriate combinations remains difficult.
To lower the chance of relapse, researchers are working on new antigens and pharmacological treatments and changing the design of CAR T cells. Maintenance therapy and stronger medications in place of traditional chemotherapy regimens can also help enhance treatment outcomes. To advance CAR T-cell therapy, bioengineering, fundamental mechanistic research, and clinical trials are required. CRISPR-Cas9 technology enables genome-wide screening for new genes that can improve CAR T-cell resistance and capacities.
CAR T-Cell Therapy Market Top Companies
- Johnson & Johnson Services, Inc.
- JW Therapeutics
- Bristol-Myers Squibb Company
- Lonza
- Novartis
- Aurora Biopharma
- Gilead Sciences
- Cartesian Therapeutics, Inc.
- ALLOGENE THERAPEUTICS
- Curocell Inc
Recent Developments
- In June 2024, A study published in Nature Medicine found that a chimeric antigen receptor (CAR) T cell therapy developed by City of Hope®, a cancer research organization based in the United States, can effectively treat advanced prostate cancer patients with minimal side effects and promising therapeutic activity, despite the challenges associated with treating prostate cancer with immunotherapy.
- In June 2024, research published in The New England Journal of Medicine discovered that second tumors following chimeric antigen receptor (CAR) T-cell treatment are uncommon after investigating the prevalence of second tumors in 724 individuals from a single institution.
- In June 2024, Shanghai First Song Therapeutics is developing Anti-HER2-CAR-T Cells, which are now in Phase I for solid tumor therapy. According to GlobalData, the drug's PTSR and LoA scores are comparable to the 70% threshold for Phase I solid tumor medicines, showing promise for Phase II trials.
Segments Covered in the Report
By Drug
- Axicabtagene Ciloleucel
- Tisagenlecleucel
- Brexucabtagene Autoleucel
- Others
By Indication
- Lymphoma
- Acute Lymphocytic Leukemia
- Chronic Lymphocytic Leukemia (CLL)
- Multiple Myeloma (MM)
- Others
By End User
- Hospitals
- Cancer Treatment Centers
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- The Middle East and Africa
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/2545
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 650 460 3308
Unlocking Market Insights through Data Excellence
The "Precedence Statistics" flexible dashboard is a powerful tool that offers real-time news updates, economic and market forecasts, and customizable reports. It can be configured to support a wide range of analysis styles and strategic planning needs. This tool empowers users to stay informed and make data-driven decisions in various scenarios, making it a valuable asset for businesses and professionals looking to stay ahead in today's dynamic and data-driven world.
To Access our Premium Real-Time Data Intelligence Tool, Visit: http://www.precedencestatistics.com
About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web: https://www.precedenceresearch.com
Our Blogs:
https://www.towardshealthcare.com
https://www.towardspackaging.com
https://www.towardsautomotive.com
For the Latest Update Follow Us:
