- NeOnc’s second concurrent Phase 2 clinical study seeks further safety and preliminary evidence of biological effects of its lead drug candidate, NEO100™, on certain cancers of the brain and central nervous system.
- Generated by NeOnc’s patented NEO™ technology platform, NEO100-02™ for meningioma indications is intended to target such tumors that occur in membranes surrounding the brain and spinal cord.
- Research of NEO100 advances under FDA Investigative New Drug (IND), Orphan Drug (OD) and Fast-Track status using oral and novel intranasal (via nose) delivery methods.
- Trial highlights the ability of the NEO™ technology platform to produce novel drugs and delivery methods designed to overcome the persistent challenges in delivering chemotherapeutics through the blood-brain barrier.
WESTLAKE VILLAGE, Calif., Aug. 05, 2024 (GLOBE NEWSWIRE) -- NeOnc Technologies Holdings, Inc., a clinical-stage medical biotechnology company, has begun patient enrollment for the Phase 2 clinical trial of NEO100-02™, the company’s first of two drug candidates proceeding through four concurrent clinical trials for various indications and patient populations.
The Phase 2 study of NEO100-02 seeks further safety and preliminary evidence of the biological effects of the novel drug on patients afflicted with residual, progressive or recurrent high-grade meningioma.
Meningioma is the most common type of benign tumor that forms in the brain. They grow from the membranes that surround the brain and spinal cord, with such growth potentially pressing on the nearby brain, nerves and blood vessels. Like any tumor, it may become atypical or malignant. Meningiomas may also develop in areas of the brain which are difficult to operate upon, such as at the base of the skull.
Approximately 97 out of every 100,000 people will be diagnosed with meningioma sometime during their lifetime, according to the Cleveland Clinic, with this resulting in more than 170,000 people diagnosed with meningioma annually in the United States.
“Addressing the unique challenges posed by skull-base meningiomas, this trial is tailored to a population with limited alternative treatment options,” commented NeOnc CEO, Thomas Chen, MD, Ph.D. “The difficulty in accessing these tumors frequently results in significant neurological deficits post-surgery, and traditional radiation therapy often proves ineffective.”
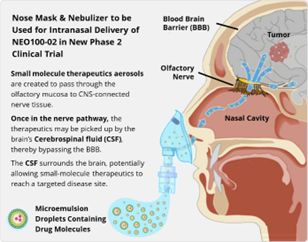
NeOnc novel intranasal delivery method for NEO100-02 offers an easier alternative for patients compared to traditional approaches, such as pills, injections and radiation. Radiation treatment and chemotherapy for high-grade gliomas are complex, time-consuming, and often result in poor prognoses.
“Such factors highlight the crucial need for developing effective therapies that are less invasive and more tolerable—particularly for the more vulnerable elderly and pediatric populations,” noted Dr. Chen.
NeOnc’s intranasal delivery method is designed to allow drugs to bypass the challenging blood-brain barrier and enter the brain through the olfactory or trigeminal nerves. Intranasal drug delivery is non-invasive and can be easily administered by or for the patient. This can potentially reduce treatment costs and improve treatment efficacy.
Intranasal delivery can also potentially enable rapid onset of action and avoidance of first-pass metabolism efficacy decrease. NEO100 intranasal delivery is anticipated to provide oncology physicians and CNS chemo-therapeutic manufacturers with the vehicle they have long sought to impact the efficacy of their treatment protocols.
Progressing under FDA Fast-Track, Orphan Drug (OD) and Investigational New Drug (IND) status, this new Phase 2 clinical trial is titled, ‘An Open-Label, Phase 2 Study of NEO100-02 in Participants with Residual, Progressive or Recurrent High-grade Meningioma.’
The new clinical trial represents NeOnc's second Phase 2 clinical trial in addition to two Phase 1 trials. The trials are for various indications and potential patient groups, and are proceeding simultaneously.
NeOnc’s other ongoing Phase 2a clinical trial for NEO100-01 is titled, ‘An Open-Label, Phase 1/2a Dose Escalation Study of Safety and Efficacy of NEO100 in Recurrent Grade IV Glioma.’ The study was recently expanded from Grade IV Astrocytoma with IDH1 mutation to include the more prevalent but equally lethal Grade III variant.
NeOnc's executive chairman, Amir Heshmatpour, commented: “We believe this initiation of our Phase 2 meningioma clinical trial for NEO100-02, along with our lead Phase 2 clinical trials for NEO100-01 also currently underway, strengthens the value of our proprietary drug development platform.
“The various indications and patient groups we can address highlight the fact we enjoy multiple ‘shots on goal’ as we pursue commercialization, which we believe also significantly increases our shareholder value.”
NEO100 is a proprietary, highly purified formulation of Perillyl Alcohol (POH) which is found in the essential oils of certain plants, such as citrus.
The biotechnology advancements generated by NeOnc's NEO™ drug development platform is the result of more than a decade of research at the University of Southern California (USC) by Dr. Chen and his medical and scientific teams.
About NeOnc Technologies Holdings
NeOnc Technologies is a privately held clinical stage life sciences company focused on the development and commercialization of central nervous system therapeutics that are designed to address the persistent challenges in overcoming the blood-brain barrier.
The company’s NEO™ drug development platform has produced a portfolio of novel drug candidates and delivery methods with patent protections extending to 2038. These proprietary chemotherapy agents have demonstrated positive effects in laboratory tests on various types of cancers and in clinical trials treating malignant gliomas. NeOnc’s NEO100™ and NEO212™ therapeutics are in Phase I and II human clinical trials, and are advancing under FDA Fast-Track and Investigational New Drug (IND) status.
The company has exclusively licensed an extensive worldwide patent portfolio from the University of Southern California consisting of issued patents and pending applications related to NEO100, NEO212, and other products from the NeOnc patent family for multiple uses, including oncological and neurological conditions.
For more about NeOnc and its pioneering technology, visit neonctech.com.
Important Cautions Regarding Forward-Looking Statements
All statements other than statements of historical facts included in this press release are "forward-looking statements" (as defined in the Private Securities Litigation Reform Act of 1995). Generally, such forward-looking statements include statements regarding our expectations, possible or assumed future actions, business strategies, events, or results of operations, including statements regarding our expectations or predictions or future financial or business performance or conditions and those statements that use forward-looking words such as "projected," "expect," "possibility" and "anticipate," or similar expressions. The achievement or success of the matters covered by such forward-looking statements involve significant risks, uncertainties, and assumptions. Actual results could differ materially from current projections or implied results.
NeOnc Technologies Holding, Inc. (the "Company") cautions that statements and assumptions made in this news release constitute forward-looking statements without guaranteeing future performance. Forward-looking statements are based on estimates and opinions of management at the time statements are made. The information set forth herein speaks only as of the date hereof. The Company and its management are under no obligation, and expressly disclaim any responsibility, to update, alter, or otherwise revise any forward-looking statements following the date of this news release, whether because of new information, future events, or otherwise, except as required by law.
NeOnc Company Contact:
Patrick Walters
Chief Operations Officer
NeOnc Technologies Holdings, Inc.
Email Contact
NeOnc Investor Relations:
Ron Both or Grant Stude
CMA Investor Relations
Tel (949) 432-7566
Email Contact
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/67913b15-033e-4b3a-9bca-32037a3009ff
