Dublin, Aug. 27, 2024 (GLOBE NEWSWIRE) -- The "United States Fabry Disease Treatment Market, By Region, Competition, Forecast and Opportunities, 2019-2029F" report has been added to ResearchAndMarkets.com's offering.
United States Fabry Disease Treatment Market was valued at USD 492.11 million in 2023 and is anticipated to project impressive growth in the forecast period with a CAGR of 6.44% through 2029
The United States Fabry Disease Treatment Market refers to the pharmaceutical and healthcare industry's efforts to develop and provide treatments for Fabry disease, a rare genetic disorder known as lysosomal storage disorder. Fabry disease is caused by the deficiency of an enzyme called alpha-galactosidase A, leading to the accumulation of a specific fatty substance in various organs and tissues. 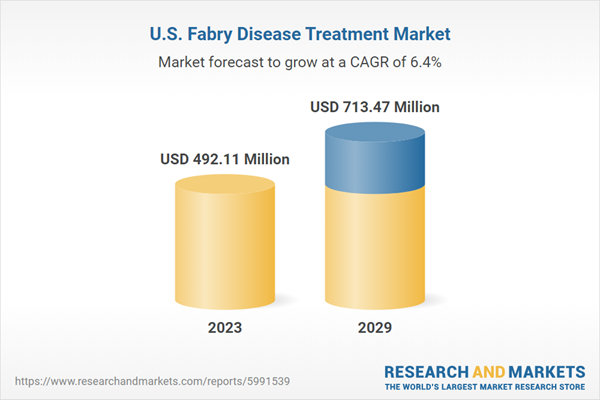
Precision Medicine and Personalized Therapies
Precision medicine is a burgeoning trend in healthcare, and it is increasingly being applied to rare diseases like Fabry disease. The concept revolves around tailoring treatments to individual patients based on their unique genetic and clinical profiles. As genomic sequencing becomes more accessible and affordable, healthcare providers can identify specific genetic mutations in Fabry disease patients. This enables the development of personalized therapies that target the root causes of the disease, potentially enhancing treatment effectiveness and reducing side effects.
Gene Therapy Advancements
Gene therapy holds immense promise for Fabry disease treatment. This innovative approach involves delivering healthy copies of the defective GLA gene into patients' cells to restore the production of the missing enzyme. Recent breakthroughs in gene therapy research have brought this treatment option closer to reality. As clinical trials progress, gene therapy may soon offer a curative solution for Fabry disease patients, significantly transforming the treatment landscape.
Small Molecule Chaperone Therapies
Small molecule chaperone therapies are emerging as a potential alternative or complementary treatment for Fabry disease. These therapies involve the use of small molecules that can stabilize and enhance the activity of the deficient enzyme. Clinical trials and research efforts are underway to develop and refine these treatments, which may provide more convenient options for Fabry disease patients compared to traditional enzyme replacement therapy (ERT).
Segmental Insights
Treatment Type Insights
Based on the category of Treatment Type, Enzyme Replacement Therapy (ERT) is anticipated to experience robust growth during the forecast period. Patients with Fabry disease can benefit from ERT, especially when initiated early, before the onset of organ damage like chronic kidney disease or cardiac fibrosis. Two major ERT options for Fabry disease, Agalsidase Beta and Agalsidase Alfa, function by mimicking the actions of alpha-galactosidase A.
This specific segment is poised for substantial expansion due to the growing demand for ERT in Fabry disease treatment and its favorable outcomes in patients. ERTs like Fabrazyme address the root cause of Fabry disease, such as mutations in the GLA gene leading to a deficiency of alpha-galactosidase. Recent studies, like the one published in the October 2022 issue of the Frontier Journal, have successfully utilized ERT to treat Fabry disease through exogenous GLA enzyme replacement. Moreover, these studies have shown the potential for significantly extended life expectancy when employing ERTs for Fabry disease, as highlighted in the May 2022 article in the IntechOpen journal. Consequently, the increasing adoption of ERTs in Fabry disease is expected to drive demand, contributing to segment growth throughout the forecast period.
Pharmaceutical companies are actively pursuing strategies to introduce new Agalsidase Beta drugs for Fabry disease treatment. A notable example is the agreement between JCR Pharmaceuticals Co., Ltd. and Sumitomo Dainippon Pharma Co., Ltd. in March 2022 for marketing Agalsidase Beta BS I.V. Infusion in Japan for Fabry disease treatment. These strategic partnerships among key industry players are poised to enhance ERT availability, further propelling market growth during the study period.
Regional Insights
The North-East region of the United States is poised to dominate the Fabry Disease Treatment Market. This region is home to some of the nation's most prestigious medical institutions and research facilities, which have been at the forefront of Fabry disease research and treatment development. These institutions attract top talent and foster groundbreaking innovations in healthcare. The North-East boasts a higher population density compared to other regions, resulting in a larger pool of potential patients and a greater demand for Fabry disease treatments. The region's robust healthcare infrastructure, coupled with a high level of insurance coverage, ensures that patients have access to cutting-edge therapies.
The North-East's strategic geographical location facilitates collaboration with pharmaceutical companies and clinical trial centers, further advancing the region's dominance in the Fabry Disease Treatment Market. In light of these factors, it is evident that the North-East region is poised to lead the way in the advancement and commercialization of treatments for Fabry disease in the United States.
Key Attributes:
| Report Attribute | Details |
| No. of Pages | 85 |
| Forecast Period | 2023 - 2029 |
| Estimated Market Value (USD) in 2023 | $492.11 Million |
| Forecasted Market Value (USD) by 2029 | $713.47 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | United States |
Report Scope:
Key Market Players
- Sanofi SA
- Takeda Pharmaceutical Co Ltd
- Amicus Therapeutics Inc
- ISU Abxis Co Ltd
- JCR Pharmaceuticals Co Ltd
- Protalix BioTherapeutics Inc
- Chiesi Farmaceutici SpA
- Freeline Therapeutics Holdings PLC
- Yuhan Corp
- M6P Therapeutics
United States Fabry Disease Treatment Market, By Treatment Type:
- Chaperone Treatment
- Enzyme Replacement Therapy
- Organ-Specific Treatment
- Substrate Reduction Therapy
United States Fabry Disease Treatment Market, By Drugs:
- Agalsidase Beta
- Migalastat
- Pipeline Drugs
United States Fabry Disease Treatment Market, By Route of Administration:
- Intravenous
- Oral
United States Fabry Disease Treatment Market, By Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
United States Fabry Disease Treatment Market, By Region:
- North-East
- Mid-west
- West
- South
For more information about this report visit https://www.researchandmarkets.com/r/6a33b9
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
