- Dramatic anti-tumor response includes complete resolution of right temporal lobe brain metastasis in patient with “Eye-Bulging” metastatic breast cancer
- Heavily pre-treated patient had failed 8 prior regimens including antibody-drug conjugate (ADC) therapy and continues to receive BriaCell treatment
PHILADELPHIA and VANCOUVER, British Columbia, Oct. 01, 2024 (GLOBE NEWSWIRE) -- BriaCell Therapeutics Corp. (Nasdaq: BCTX, BCTXW) (TSX: BCT) (“BriaCell” or the “Company”), a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care, is pleased to report dramatic anti-tumor response including complete resolution of temporal lobe breast cancer metastasis in a patient treated in the Phase 2 study of BriaCell’s Bria-IMT™ plus an immune checkpoint inhibitor regimen. The patient demonstrated an initial partial response at 2 months in the brain lesion with no detectable disease following 8 and 11 months of treatment.
The heavily pre-treated patient who had failed 8 prior regimens including ADC therapy, previously demonstrated significant reduction of her “Eye-Bulging” orbital tumor and continues treatment with the Bria-IMT™ regimen. She has completed 17 cycles of treatment and has been on BriaCell’s Phase 2 study for 12 months. Also noteworthy is a sustained drop in her tumor markers, confirming the imaging results of marked tumor reduction.
Figure 1: Bria-IMT™ regimen resulted in 100% resolution of tumor in the right temporal lobe region of the brain
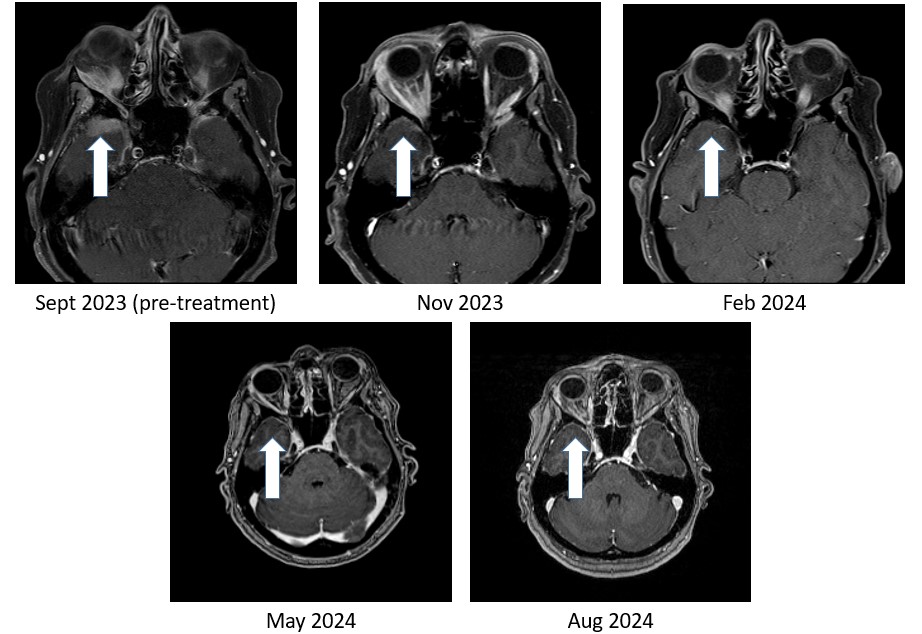
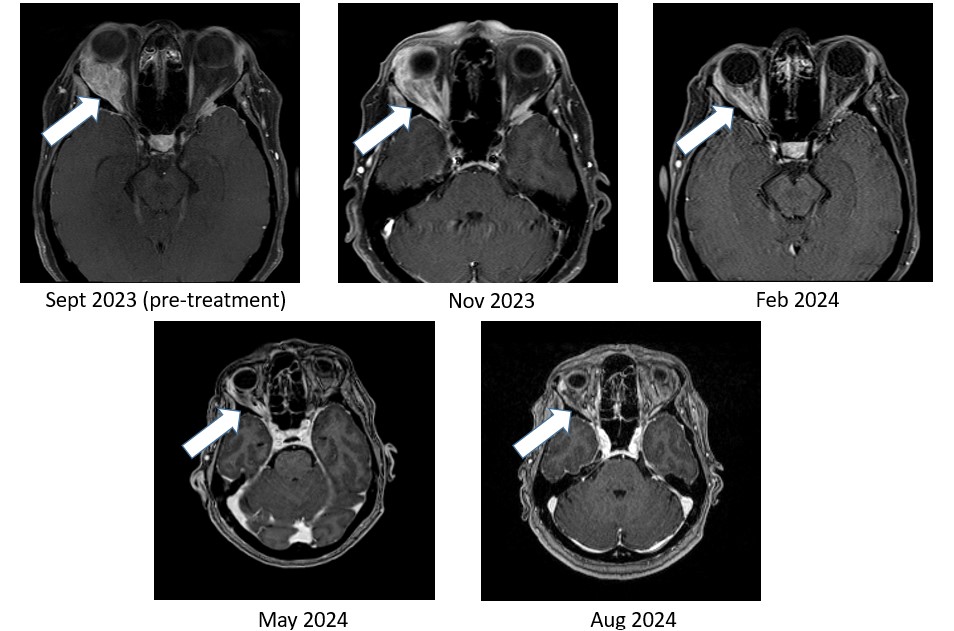
As shown in Figure 1, the right temporal lobe lesion is no longer detectable on the images taken at 8 months and 11 months on the Bria-IMT™ combination regimen. The orbital lesion has continued to shrink markedly (Figure 2). In addition, her tumor markers (blood tests that correlate with the amount of tumor in the body) remain markedly decreased from her pre-treatment levels.
Figure 2: Bria-IMT™ regimen resulted in near complete resolution of breast cancer tumor in the right orbit (behind the eye)
“Bria-IMT™’s potential therapeutic impact is unprecedented in metastatic breast cancer (MBC) in a brain metastasis setting. Our clinical findings, demonstrating significant tumor shrinkage in metastatic brain legions, may transform the way we treat MBC patients with brain metastasis, and offers hope to cancer patients and their families fighting this devastating disease,” stated Dr. William V. Williams, BriaCell’s President and CEO. “These results support Bria-IMT™ as a potential new therapeutic option for MBC patients with brain metastasis. We look forward to evaluating the brain metastasis patient subgroup in our ongoing pivotal Phase 3 study in metastatic breast cancer.”
“We believe that the complete tumor resolution in this patient with brain metastasis, plus other cases of significant anti-cancer clinical responses in our Phase 2 MBC patients with brain metastasis, highlight the potential application of Bria-IMT™ in treating similar MBC patients,” commented Dr. Giuseppe Del Priore, BriaCell’s Chief Medical Officer. “The protracted time on therapy, now one year, attests to the excellent tolerability of the Bria-IMT™ regimen in combination with an immune checkpoint inhibitor which is being used in our pivotal Phase 3 study.”
About BriaCell Therapeutics Corp.
BriaCell is a clinical-stage biotechnology company that develops novel immunotherapies to transform cancer care. More information is available at https://briacell.com/.
Safe Harbor
This press release contains “forward-looking statements” that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this press release are forward-looking statements. Forward-looking statements contained in this press release may be identified by the use of words such as “anticipate,” “believe,” “contemplate,” “could,” “estimate,” “expect,” “intend,” “seek,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “target,” “aim,” “should,” “will,” “would,” or the negative of these words or other similar expressions, although not all forward-looking statements contain these words. Forward-looking statements, including those about BriaCell replicating positive data in its ongoing pivotal Phase 3 study; BriaCell’s Bria-IMT™ regimen bringing relief to cancer patients whose medical needs remain unmet; and the Bria-IMT™ regimen becoming a therapeutic option for metastatic breast cancer patients, are based on BriaCell’s current expectations and are subject to inherent uncertainties, risks, and assumptions that are difficult to predict. Further, certain forward-looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully under the heading “Risks and Uncertainties” in the Company’s most recent Management’s Discussion and Analysis, under the heading “Risk Factors” in the Company’s most recent Annual Information Form, and under “Risks and Uncertainties” in the Company’s other filings with the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission, all of which are available under the Company's profiles on SEDAR+ at www.sedarplus.ca and on EDGAR at www.sec.gov. Forward-looking statements contained in this announcement are made as of this date, and BriaCell Therapeutics Corp. undertakes no duty to update such information except as required under applicable law.
Neither the Toronto Stock Exchange nor its Regulation Services Provider (as that term is defined in the policies of the Toronto Stock Exchange) accepts responsibility for the adequacy or accuracy of this release.
Contact Information
Company Contact:
William V. Williams, MD
President & CEO
1-888-485-6340
info@briacell.com
Media Relations:
Jules Abraham
CORE IR
julesa@coreir.com
Investor Relations Contact:
CORE IR
investors@briacell.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/0a328ad2-c48d-43bf-ae04-0b69da4d3f3f
https://www.globenewswire.com/NewsRoom/AttachmentNg/eea9560e-c63a-4001-a5b7-6a07caf3c91b
