New York, USA, Oct. 08, 2024 (GLOBE NEWSWIRE) -- VEGFR-2 Antagonists Clinical Trial Pipeline Insights Featuring 25+ Companies | DelveInsight
VEGFR2 (vascular endothelial growth factor receptor 2) antagonist is an antineoplastic agent that blocks the binding of natural VEGF ligands, which are secreted by solid tumors to promote angiogenesis and enhance tumor blood supply. Continuous research and development in oncology has led to a better understanding of tumor angiogenesis, the process by which tumors develop their blood supply. This has spurred the development and adoption of VEGFR-2 antagonists as targeted therapies, driving market growth.
DelveInsight’s 'VEGFR-2 Antagonists Pipeline Insight 2024' report provides comprehensive global coverage of pipeline VEGFR-2 antagonists in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the VEGFR-2 antagonists pipeline domain.
Key Takeaways from the VEGFR-2 Antagonists Pipeline Report
- DelveInsight’s VEGFR-2 antagonists pipeline report depicts a robust space with 25+ active players working to develop 25+ pipeline VEGFR-2 antagonists.
- Key VEGFR-2 antagonist companies such as AiViva BioPharma, Incyte Corporation, Avalyn Pharmaceuticals, Bayer, Russian Pharmaceutical Technologies, Advenchen Laboratories, Jiangsu Hengrui Medicine, Deciphera Pharmaceuticals, Ocular Therapeutix, Mirati Therapeutics, Exelixis/Ipsen, CSPC Pharmaceutical Group, Chipscreen Biosciences, HUTCHMED, Eli Lilly and Company, Taiho Pharmaceutical, TiumBio, and others are evaluating new VEGFR-2 antagonist drugs to improve the treatment landscape.
- Promising pipeline VEGFR-2 antagonists such as Lenvatinib, Pemigatinib, Nintedanib, Regorafenib, RPT-835, Lucitanib, Famitinib, Ripretinib, Axitinib, Sitravatinib, Cabozantinib, Simmitinib, Ibcasertib, Surufatinib, LY 2874455, TAS 12, TU2218, and others are under different phases of VEGFR-2 antagonists clinical trials.
- In March 2024, TiumBio Co., Ltd. announced that it had submitted an Investigational New Drug (IND) application to the Korean Ministry of Food and Drug Safe (MFDS) for a Phase IIa study of TU2218.
- In June 2024, Eisai announced a label update for LENVIMA, the orally available multiple receptor tyrosine kinase inhibitor discovered by Eisai, in the United States to include clinical efficacy data for the first-line treatment of advanced non-clear cell renal cell carcinoma (nccRCC). This update is based on data from KEYNOTE-B61, a Phase II, single-arm trial evaluating KEYTRUDA® (pembrolizumab), Merck's anti-PD-1 therapy, plus LENVIMA for the first-line treatment of adult patients with advanced nccRCC.
- In May 2023, Mirati Therapeutics announced that the SAPPHIRE study did not meet its primary endpoint of overall survival at the final analysis. SAPPHIRE is a Phase III study evaluating sitravatinib in combination with nivolumab (OPDIVO®)1 versus docetaxel in patients with second or third-line advanced non-squamous non-small cell lung cancer.
Request a sample and discover the recent advances in VEGFR-2 antagonist drugs @ VEGFR-2 Antagonists Pipeline Report
The VEGFR-2 antagonists pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage VEGFR-2 antagonist drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the VEGFR-2 antagonists clinical trial landscape.
VEGFR-2 Antagonists Overview
Vascular Endothelial Growth Factor Receptor 2 (VEGFR-2) antagonists are a class of therapeutic agents designed to inhibit the activity of VEGFR-2, a receptor crucial for angiogenesis, the process through which new blood vessels form from pre-existing ones. This receptor is predominantly expressed on endothelial cells and plays a significant role in tumor growth, metastasis, and various other pathological conditions associated with abnormal blood vessel formation. By targeting VEGFR-2, these antagonists aim to disrupt the signaling pathways that promote angiogenesis, thereby limiting tumor growth and improving outcomes in diseases where abnormal blood vessel proliferation is a key factor.
In clinical practice, VEGFR-2 antagonists are used in the treatment of several types of cancers, including renal cell carcinoma, colorectal cancer, and non-small cell lung cancer. These drugs can be used alone or in combination with other treatments to enhance therapeutic efficacy. While effective, their use can be associated with side effects such as hypertension, proteinuria, and bleeding disorders, which necessitate careful monitoring and management. Ongoing research continues to refine these therapies, exploring novel VEGFR-2 inhibitors and combination approaches to improve patient outcomes and minimize adverse effects.
Find out more about VEGFR-2 antagonist drugs @ VEGFR-2 Antagonists Analysis
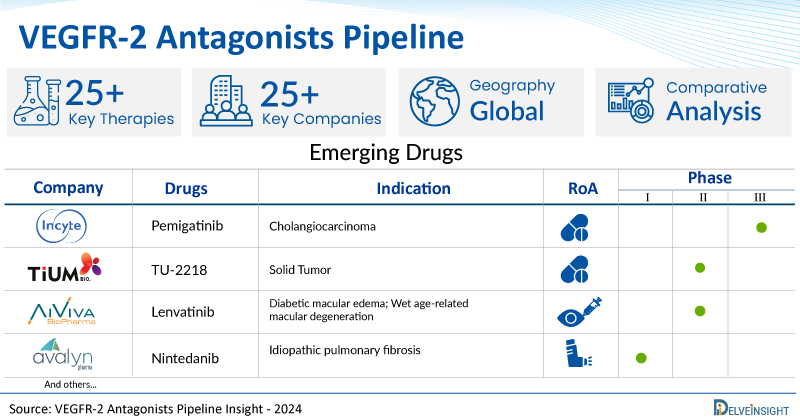
A snapshot of the Pipeline VEGFR-2 Antagonists Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| Pemigatinib | Incyte Corporation | Phase III | Cholangiocarcinoma | Oral |
| TU-2218 | TiumBio | Phase I/II | Solid Tumor | Oral |
| Lenvatinib | AiViva BioPharma | Phase I/II | Diabetic macular edema; Wet age-related macular degeneration | Intravitreal |
| Nintedanib | Avalyn Pharmaceuticals | Phase I | Idiopathic pulmonary fibrosis | Inhalation |
| RPT-835 | Russian Pharmaceutical Technologies | Phase I | Gastric Cancer | Intravenous |
Learn more about the emerging VEGFR-2 antagonists @ VEGFR-2 Antagonists Clinical Trials
VEGFR-2 Antagonists Therapeutics Assessment
The VEGFR-2 antagonists pipeline report proffers an integral view of the emerging VEGFR-2 antagonists segmented by stage, product type, molecule type, and route of administration.
Scope of the VEGFR-2 Antagonists Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Infusion, Intradermal, Intramuscular, Intranasal, Intravaginal, Oral, Parenteral, Subcutaneous, Topical
- Therapeutics Assessment By Molecule Type: Vaccines, Monoclonal antibody, Peptides, Polymer, Small molecule
- Key VEGFR-2 Antagonists Companies: AiViva BioPharma, Incyte Corporation, Avalyn Pharmaceuticals, Bayer, Russian Pharmaceutical Technologies, Advenchen Laboratories, Jiangsu Hengrui Medicine, Deciphera Pharmaceuticals, Ocular Therapeutix, Mirati Therapeutics, Exelixis/Ipsen, CSPC Pharmaceutical Group, Chipscreen Biosciences, HUTCHMED, Eli Lilly and Company, Taiho Pharmaceutical, TiumBio and others
- Key VEGFR-2 Antagonists Pipeline Therapies: Lenvatinib, Pemigatinib, Nintedanib, Regorafenib, RPT-835, Lucitanib, Famitinib, Ripretinib, Axitinib, Sitravatinib, Cabozantinib, Simmitinib, Ibcasertib, Surufatinib, LY 2874455, TAS 12, TU2218, and others
Dive deep into rich insights for new VEGFR-2 antagonists, visit @ VEGFR-2 Antagonists Drugs
Table of Contents
| 1. | VEGFR-2 Antagonists Pipeline Report Introduction |
| 2. | VEGFR-2 Antagonists Pipeline Report Executive Summary |
| 3. | VEGFR-2 Antagonists Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | VEGFR-2 Antagonists Clinical Trial Therapeutics |
| 6. | VEGFR-2 Antagonists Pipeline: Late-Stage Products (Pre-registration) |
| 7. | VEGFR-2 Antagonists Pipeline: Late-Stage Products (Phase III) |
| 8. | VEGFR-2 Antagonists Pipeline: Mid-Stage Products (Phase II) |
| 9. | VEGFR-2 Antagonists Pipeline: Early-Stage Products (Phase I) |
| 10. | VEGFR-2 Antagonists Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the VEGFR-2 Antagonists Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the VEGFR-2 Antagonists Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the VEGFR-2 antagonists pipeline therapeutics, reach out @ VEGFR-2 Antagonists Therapeutics
Related Reports
Glioblastoma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key glioblastoma companies including Laminar Pharmaceuticals, BioMimetix, Enterome, Genenta Science, Medicenna Therapeutics, Bayer, Chimerix, Aivita Biomedical, Denovo Biopharma, Northwest Biotherapeutics, MedImmune, DNAtrix, Imvax, MimiVax, CNS Pharmaceuticals, Oblato, Enterome, VBI Vaccines, among others.
Glioblastoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key glioblastoma companies, including Denovo Biopharma, Cantex Pharmaceuticals, CNS Pharmaceuticals, CANbridge Pharmaceuticals, Vaximm, Inovio Pharmaceuticals, Mustang Bio, Bullfrog AI Holdings, Cantex, Chimeric Therapeutics, Philogen, Boehringer Ingelheim, Photonamic GmbH, Berg Pharma, Beyond Bio, Genenta Science, Polaris Pharmaceuticals, Telix Pharmaceuticals, Shanghai Simnova Biotechnology, NEONC Technologies, among others.
Age-related Macular Degeneration Market
Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, market share of the individual therapies, and key AMD companies including Regeneron Pharmaceuticals, Novartis, Roche, Opthea Limited, Kodiak Sciences Inc., REGENXBIO, Alkahest Inc, Graybug Vision, Ribomic USA Inc, Outlook Therapeutics, Inc., Unity Biotechnology, Inc, PanOptica, Inc., Clearside Biomedical, Alexion Pharmaceuticals, AstraZeneca, Evergreen Therapeutics, Alkeus Pharmaceuticals, Stealth BioTherapeutics, CellCure Neurosciences, Regenerative Patch Technologies, Allegro Ophthalmics, Annexon Biosciences, NGM Biopharmaceuticals, Ionis Pharmaceuticals, Apellis Pharmaceuticals, Iveric Bio, Gyroscope Therapeutics, Luxa Biotechnology, Gemini Therapeutics, among others.
Dry Age-related Macular Degeneration Pipeline
Dry Age-related Macular Degeneration Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key dry AMD companies, including Alkeus Pharmaceuticals, Dobecure, Iveric Bio, Mitotech, Stealth BioTherapeutics, Allergo Ophthalmics, Gyroscope Therapeutics, Gemini Therapeutics, Luxa Biotechnology, CHABiotech CO., Ltd, Regenerative Patch Technologies, Lineage Cell Therapeutics, Janssen Research & Development, Belite Bio, Katairo, Cognition Therapeutics, Eye Co Pty Ltd, Applied Genetic Technologies Corporation (AGTC), Aviceda Therapeutics, Kodiak Sciences, Ascentage Pharma, OliX Pharmaceutical, Nanoscope Therapeutics, Inc., Biophytis, Eyestem, Eyevensys, SanBio, Beta Therapeutics, MacRegen, Amarna Therapeutics, MeiraGTx, Cognition Therapeutics, Ocugen, Catalyst Biosciences, Endogena Therapeutics, Oculogenex, Clover Therapeutics, Retrotope, Stuart Therapeutics, Surrozen, 4D Pharma, Ocular Therapeutix, among others.
Wet Age-related Macular Degeneration Market
Wet Age-related Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Wet Age-related Macular Degeneration companies including EyePoint Pharmaceuticals, Inc., AbbVie, Caregen Co. Ltd., Exegenesis Bio, Shanghai Henlius Biotech, Skyline Therapeutics, 4D Molecular Therapeutics, Ocugenix Corporation, Adverum Biotechnologies, Inc., Ashvattha Therapeutics, Inc., AiViva BioPharma, Inc., Ocular Therapeutix, Inc., Clearside Biomedical, Inc., Hoffmann-La Roche, Kyowa Kirin, Inc., Opthea Limited, AffaMed Therapeutics Limited, EyeBiotech Ltd., Novartis, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
