New York, Nov. 25, 2024 (GLOBE NEWSWIRE) -- Market Overview
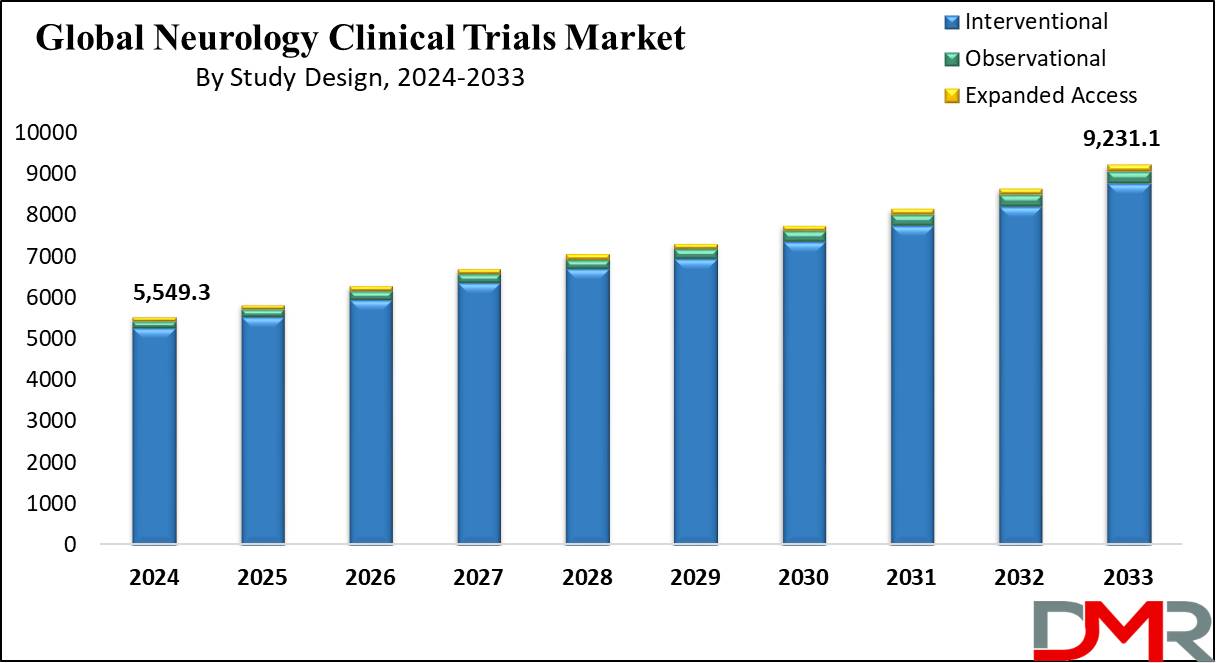
The Global Neurology Clinical Trials Market size is expected to reach USD 5,549.3 million by 2024 and it is further anticipated to reach a market value of USD 9,231.2 million by 2033 at a CAGR of 5.8% from 2024 to 2033.
The neurology clinical trials market encompasses innovative treatments against neurologic conditions like Alzheimer's, Parkinson's, and Huntington's diseases. With increased funding for research, stem cell therapies becoming more mainstream genetic advancements as well as AI technology in trial management emerging as key drivers in driving market expansion, along with phase II and phase III trials featuring gene therapies or disease-modifying medications along with numerous innovations set to emerge over the coming period..
Due to the emergence of recent innovations, the neurology clinical trials market size is very likely to grow visibly during the forecast period.
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/neurology-clinical-trials-market/request-sample/
The US Neurology Clinical Trials Market
The US neurology clinical trials market with an estimated value of USD 2,244.8 million in 2024 is projected to increase at a CAGR of 5.4% until reaching USD 3,618.9 million by 2033.
The U.S. neurology clinical trials market is among the biggest hubs for neurological research, considering the high prevalence of conditions such as Alzheimer's and Parkinson's. Trends are an increase in Phase II and III trials for gene therapies and personalized medicine.
In parallel with that, there is an increasing use of AI-driven platforms that will help in easing patient recruitment and trial efficiency. Major happenings in strategic partnerships among biopharma companies and CROs are to bring improvement in trial outcomes.
The United States is in the lead when it comes to neurology clinical trials, owing to an integrated healthcare infrastructure and adequate funding regarding neurological R&D.
Important Insights
- Global Market Value: This market is projected to reach USD 5,549.3 million in 2024, with an expected increase to USD 9,231.1 million by 2033.
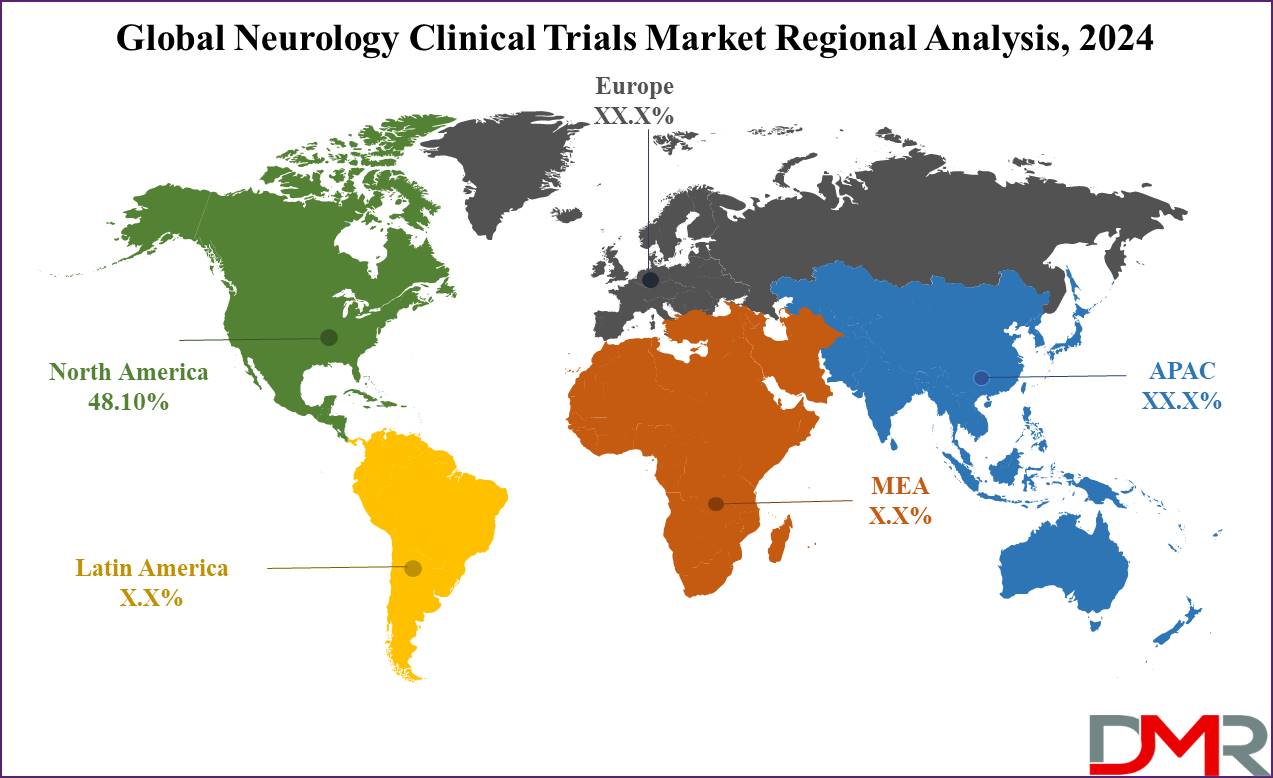
- Regional Analysis: North America is anticipated to capture the largest market share in this market, accounting for approximately 48.10% in 2024.
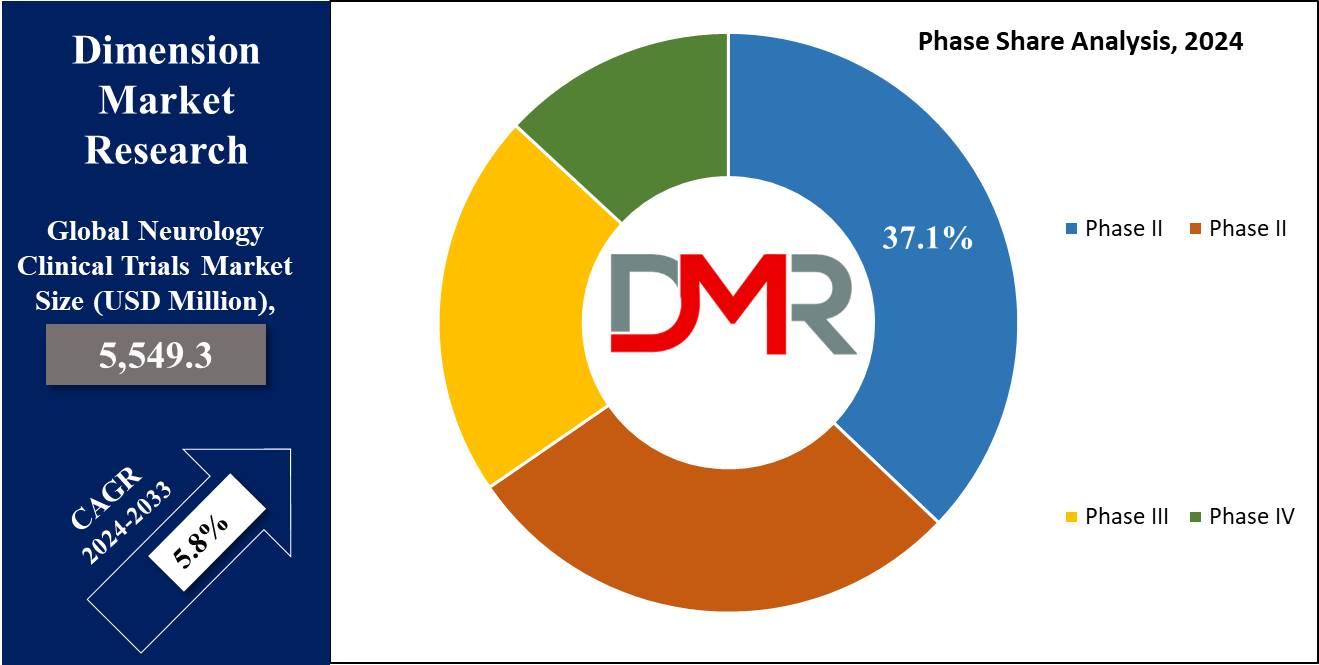
- Phase Segment Analysis: Phase II clinical trials are forecasted to dominate this segment, holding a market share of 37.1% in 2024.
- Study Design Segment Analysis: Interventional study designs are expected to lead the market, comprising 94.9% of the market share in 2024.
- Key Players: Major players in the global neurology clinical trials market include Novartis, Covance, Medpace, Charles River Laboratories, among others.
- Global Growth Rate: The market is anticipated to grow at a CAGR of 5.8% during the forecast period.
- US Market Value: The US neurology clinical trials market is estimated to reach USD 2,244.8 million in 2024, with an expected growth to USD 3,618.9 million by 2033.
Latest Trends
- Larger Emphasis on Gene Therapies: The number of Phase II gene therapies focused on rare neurological disorders is significantly increasing, especially for the treatment of Huntington's disease-a clear indication of new innovative choices that the industry is making.
- Adoption of AI-Powered Platforms: Increasing integration of AI technologies in trial management is expected to result in better recruitment and operationally efficient performance, which will reduce timelines in neurological clinical trials.
- Growing Collaborations: Expanding collaborations by biopharma firms with CROs are affordable means of conducting highly complex trials, thus accelerating the development of therapies and enhancing market access for new treatments.
Neurology Clinical Trials Market: Competitive Landscape
The neurology clinical trials constitute an extremely competitive market with the involvement of key players including but not limited to Biogen, Pfizer, Roche, Novartis, and Eli Lilly. Companies are targeted through gene therapies and disease-modifying treatments for neurological disorders such as Alzheimer's and Parkinson's.
Among the contract research organizations involved in the management of clinical trials, the leading ones include IQVIA and PRA Health Sciences. Competition is driven by increased investment in R&D, technological improvements, and deals between biotech companies and CROs. The pipeline for treatments pertaining to rare neurological disorders is also on the rise, thus driving competition in the market.
Some of the prominent market players:
- Novartis
- Covance
- Med pace
- Charles River Laboratories
- Syneous Health
- Icon Plc.
- GlaxoSmithKline
- Aurora healthcare
- Biogen
- IQVIA
- Other Key Players
Transform your business approach with strategic insights from our report. Get in touch to request our brochure today! : https://dimensionmarketresearch.com/report/neurology-clinical-trials-market/download-reports-excerpt/
Neurology Clinical Trials Market Scope
| Report Highlights | Details |
| Market Size (2024) | USD 5,549.3 Mn |
| Forecast Value (2033) | USD 9,231.1 Mn |
| CAGR (2024-2033) | 5.8% |
| North America Revenue Share (2024) | 48.10% |
| The US Market Size (2024) | USD 2,244.8 Mn |
| Historical Data | 2018 - 2023 |
| Forecast Data | 2024 - 2033 |
| Base Year | 2023 |
| Estimate Year | 2024 |
| Segments Covered | By Phase, By Study Design, By Indication |
| Regional Coverage | North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
Market Analysis
Phase II is projected to dominates the neurology clinical trials market with 37.1% of market share in 2024. Phase II holds the largest share in the neurology clinical trials as it investigates the effectiveness of new therapies a major factor in drug development. These trials compare the harms and benefits of new treatments and aim at determining the right dose for the drugs or the right beneficial treatment regimen.
The validity of using animal models in neurological diseases also comes out clearly in Phase II trial, where patient response to disease progression is well understood. As more pharmaceutical companies turn to gene therapies and disease-modifying drugs, Phase II remains ahead due to its critical function of transferring early-stage research to Phase III.
Neurology Clinical Trials Market Segmentation
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Expanded access
By Indication
- Epilepsy
- Parkinson’s Disease
- Huntington’s Disease
- Stroke
- Traumatic brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle regeneration
- Other Indications
Purchase the Competition Analysis Dashboard Today: https://dimensionmarketresearch.com/checkout/neurology-clinical-trials-market/
Growth Drivers
- Rising Prevalence of Neurological Diseases: The high prevalence of diseases like Alzheimer’s and Parkinson’s also place market pressure to seek out advanced therapies, as well as widen clinical trials’ range.
- Technological Advancements: Prolific advancement in technologies in the drug development such as genetic engineering, gene therapy and stem cell research has greatly improved the rate of conducting clinical trials in neurology.
- Increased Government Funding: An improved government support and funding for the neurological disorders are encouraging the frequent trials across the world from clinical perspective with increased development.
Restraints
- High Costs of Complex Trials: Intricate clinical trials involving the neurological disorder, to begin with, and even more so for sophisticated treatments such as gene modification, are expensive, and are suggestive of restricting the number of trials that can be practically attempted.
- Regulatory Hurdles: Delays resulting from stringent regulatory environments in the approval of new treatments are evident, especially in areas of gene and stem cell research where the market approval can greatly be slowed down.
- Patient Recruitment Challenges: Patients are sometimes hard to reach for trial participation in clinical trials focusing on rare neurological disorders hence limiting the trials and therefore the overall market.
Growth Opportunities
- Expanding AI and Machine Learning: AI and machine learning have reformed selective aspects of neurology clinical trials such as patient recruitment and monitoring, and have major potential for further innovation regarding the subject.
- Rise of Personalized Medicine: Increasing importance is attached to developing a differentiated approach in the given field, providing targeted treatment of multiple diseases using new trials, enlarging the market at the same time.
- Focus on Neurodegenerative Diseases: High unmet needs conditions such as ALS and Huntington’s disease provide growth driven by focused therapy and unique clinical trial development.
Regional Analysis
North America is projected to dominate the neurology clinical trials market with 48.10% of market share in 2024. North America is considered to dominate the neurology clinical trials market due to its first-rate health infrastructure, extensive R&D investments in the field, and high prevalence of neurological experience, such as Alzheimer's disease.
Advanced technological integrations of trials-geographical benefits include AI for recruitment and data analysis. The headquarters of leading biopharmaceutical companies and contract research organizations within North America are driving these clinical trials.
Government funding and supportive regulatory frameworks for neurological research are also favoring the market. The United States has been at the forefront, housing numerous ongoing and various neurology clinical trials across the globe.
Click to Request Sample Report and Drive Impactful Decisions: https://dimensionmarketresearch.com/report/neurology-clinical-trials-market/request-sample/
By Region
North America
- The U.S.
- Canada
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Browse More Related Reports
- Hearth Market is expected to value USD 13.6 billion in 2024, and it is further anticipated to reach a market value of USD 24.5 billion by 2033 at a CAGR of 6.8 %.
- Sinus Dilation Devices Market size is estimated to reach a value of USD 3.5 billion in 2024 and is expected to grow at a compound annual growth rate (CAGR) of 10.0% for the forecasted period (2024-2033).
- Companion Diagnostics Market size was valued USD 8.0 billion in 2023 which is further expected to reach a market value of USD 26.0 billion in 2033 at a CAGR of 12.5%.
- Cell Therapy Market size is expected to reach a value of USD 7.0 billion in 2024, and it is further anticipated to reach a value of USD 32.5 billion by 2033 at a CAGR of 18.6%.
- Cell Culture Market size is expected to reach a value of USD 31.4 billion in 2024, and it is further anticipated to reach a market value of USD 80.3 billion by 2033 at a CAGR of 11.0%.
- CDMO (Contract Development and Manufacturing Organization) Market size is estimated to reach USD 272.4 billion in 2024 and is expected to reach USD 530.3 billion by 2033, growing at a CAGR of 7.7% during the forecast period (2024-2033).
- CAR-T Cell Therapy Market size is expected to have a value of USD 7.1 billion in 2024, and it is further predicted to reach a market value of USD 85.8 billion by 2033 at a CAGR of 32.0%.
- Biopharmaceutical Market was reached a value of USD 436.7 billion in 2023, and it is further anticipated to reach a market value of USD 1,829.2 billion by 2033 at a CAGR of 15.4%.
- Clinical Trials Market size is expected to reach a market value of USD 96.8 billion in 2024 which is further expected to reach USD 174.3 billion in 2033 at a CAGR of 6.8%.
- Blood Pressure Monitoring Device Market is expected to reach a value of USD 5.7 billion in 2024, and it is further anticipated to reach a market value of USD 13.2 billion by 2033 at a CAGR of 9.8%.
Recent Developments in the Neurology Clinical Trials Market
- October 2024: Biogen announces promising Phase II results for its Huntington’s disease gene therapy, showcasing potential effectiveness and safety, setting a path for further clinical exploration.
- September 2024: Pfizer initiates a Phase III clinical trial targeting amyloid plaques in Alzheimer’s disease, aiming to validate therapeutic efficacy and move towards potential market approval.
- August 2024: Roche starts a Phase II trial evaluating its novel gene therapy for Parkinson's disease, signaling ongoing commitment to addressing unmet needs in neurological disorders.
- July 2024: IQVIA partners with Novartis to launch an AI-powered trial management platform, designed to streamline processes and enhance operational efficiency in neurology clinical trials.
- June 2024: AstraZeneca completes its Phase II trial for multiple sclerosis, demonstrating reduced relapse rates, thereby supporting the potential of its treatment approach in the market.
- May 2024: Eli Lilly begins a Phase II trial for frontotemporal dementia, expanding its research portfolio in neurological disorders, aiming to address critical treatment gaps in this area.
About Dimension Market Research (DMR):
Dimension Market Research (DMR) is a market research and consulting firm based in India & US, with its headquarters located in the USA (New York). The company believes in providing the best and most valuable data to its customers using the best resources analysts into work, to create unmatchable insights into the industries, and markets while offering in-depth results of over 30 industries, and all major regions across the world.
We also believe that our clients don’t always want what they see, so we provide customized reports as well, as per their specific requirements to create the best possible outcomes for them and enhance their business through our data and insights in every possible way.
