Turning Science Fiction
into Science Fact
Universally implantable regenerative human tissues, at commercial scale
Humacyte® is pioneering
a platform that enables the investigation, development, and manufacture of
bioengineered human tissues and organs that are designed to be universally implantable,
off-the-shelf, and regenerative, with the goal of treating a wide variety of diseases,
injuries, and chronic conditions.

Our latest news

FDA Completed Review of Symvess™ product batch release and Humacyte is now authorized to commercially ship product

Humacyte Announces FDA Approval of Symvess™ (acellular tissue engineered vessel-tyod) for the Treatment of Extremity Vascular Trauma

Humacyte Acellular Tissue Engineered Vessel (ATEV™) Meets Primary Endpoints
in V007 Phase 3 Clinical Trial in Arteriovenous Access for Hemodialysis

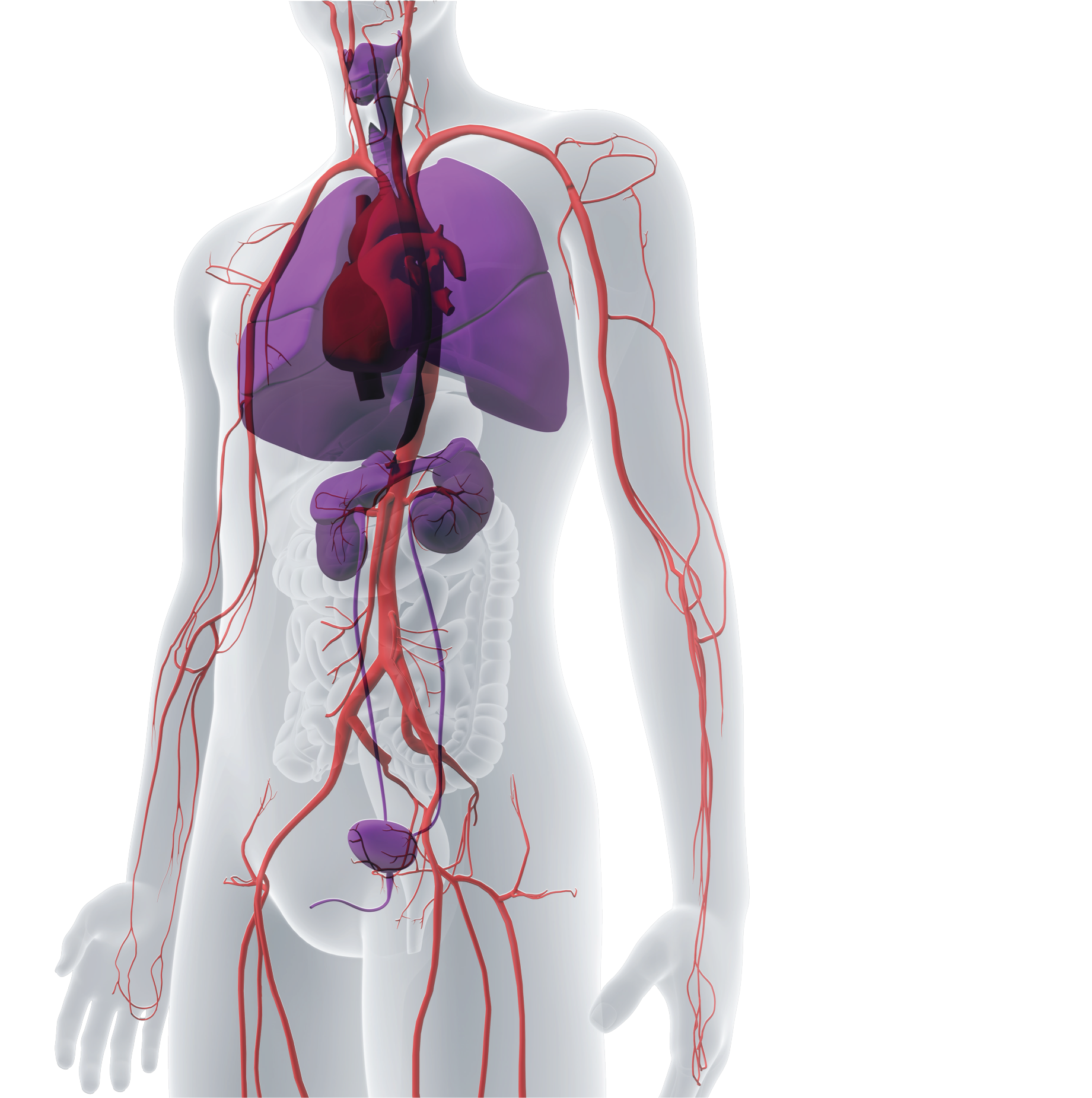
The Platform
Our first-of-its-kind bioengineering platform is designed to make tissue repair and replacement accessible to a broader patient population.
We’ve pioneered a scalable bioengineering platform that transforms human cells into universally implantable human tissues being investigated for the treatment of injury, disease, and chronic conditions, across a wide range of clinical needs. The goal is to provide regenerative biologic tissues that are available off-the-shelf, with low rates of infection, and universally implantable.
Learn more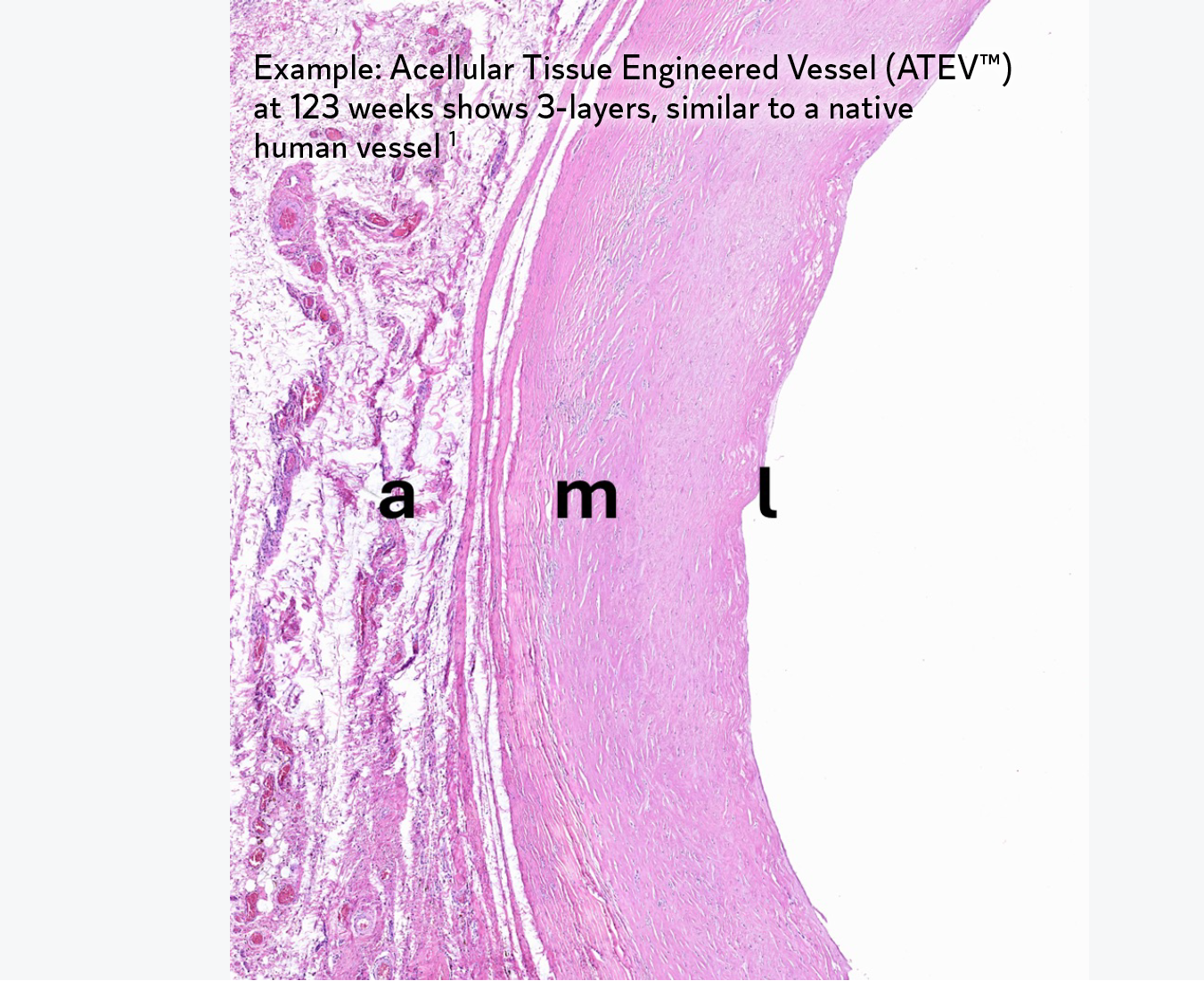

The Science
Our tissue engineered platform is designed to harness the body’s own natural processes.
During the decellularization stage of the proprietary manufacturing process, cells are removed from bioengineered tissues while key proteins are retained. These proteins signal the patient’s own cells to bind to the extracellular matrix (ECM), and proliferate.1 Over time, the bioengineered tissue becomes the patient’s own tissue.1
Learn moreCore areas of focus
We are currently building an extensive portfolio and pipeline of investigational product candidates to address a broad range of clinical needs - all built on the foundational science of our Acellular Tissue Engineered Vessel platform.
Learn moreBiologics License Agreement (BLA) approved by U.S. FDA December, 2024
These are investigational uses that have not been approved by any health authority. Safety and efficacy have not been established and there is no guarantee that pipeline products or investigational uses will receive approval from health authorities.
- Kirkton RD, et al. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11(485):eaau6934.